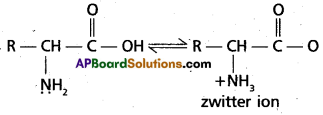
Access to a variety of AP Inter 2nd Year English Model Papers Set 10 allows students to familiarize themselves with different question patterns.
AP Inter 2nd Year English Model Paper Set 10 with Solutions
Time : 3 Hours
Max. Marks : 100
SECTION – A
I.Annotate ANY TWO of the following in about 10-15 lines each : (2 × 4 = 8)
Question 1.
Studies serve for delight, for ornament and for ability.
Answer:
Context: This line is taken from the essay “Of Studies” written by Francis Bacon. He was called the Father of English Essay. This line is about the benefits of studies.
Explanation : We get delight when we read a book. When we are at leisure, we read and enjoy. Reading enriches our capacity of communication and judgement. Thus it makes us efficient and it is like an ornament. Effective thinking and perfect knowledge are possible by studies. Many people take the advice of a man who studies well.
General Relevance: The short sentence, has got great meaning. Bacon’s sentences are aphoristic and are filled with sensitive views.
Question 2.
Every work must necessarily be a mixture of good and evil yet we are commanded to work incessantly.
Answer:
Context: This passage is taken from the essay “The Secret of Work” written by the great thinker Swami Vivekananda. In this sentence he writes about the need of regular work. The secret of work is discussed here.
Explanation : Vivekananda gives prominence to work. Every work has got the two faces, the good and the bad. We have to pick up good and work without any stop. Through good work, good effect will come into being. The good things coming out of one’s character belong to our Samskara. The senses are controlled and there is no attachment with the evil. The secret of work is doing the things without any delay.
General Relevance : According to Vivekananda, the secret of work, is doing it without any stop. Love drives the man into action and the secret of work lies in continuity.
Question 3.
My gloomy thoughts probably stem from an accident I had few years ago.
Answer:
Context: This sentence is ta^en from the essay “In Celebration of Being Alive” written by Dr. Christian Barnard. When the writer thought about the suffering prevailing in the world, he remembered an accident in his life. He described the accident in the essay.
Explanation :.His thoughts were gloomy but his suffering was alive in his mind. As he and his wife were crossing the street, they were caught in the accident. They got treatment at the hospital. But it was strange for him, why he should get such a punishment, by God. His anguish was great, the unexpected suffering, according to the narrator, restricted him from the service he *had to render to the patients.
General Relevance : The suffering stopped the services of the doctor. He had broken ribs. So, the narrator was angry with God, for this situation.

Question 4.
Meritocracy by definition means that we cannot let personal prejudices affect our evaluation of an individual’s performance.
Answer:
Context: This passage is taken from the essay “Learning from the West” written by N.R.Narayanh Murthy. The writer was a great Industrialist and a Writer. Here he writes about the virtues, one should possess in life.
Explanation : In the West people evaluate based on merit. No k personal prejudices or interests come in the way. In the West right from the young age, they are accustomed to this way of living. So, the Indian,-has to give respect to this clean thinking. It enhances our respect in the society. Meritocracy is a great virtue.
General Relevance : Personal interests should not come in the way, one gives respect to the merits. This will add to the character of an individual.
II. Annotate ANY TWO of the following in about 10-15 lines each : (2 × 4 = 8)
Question 1.
It shall be still in strictest measure ev’n,
To that same lot, however mean or high.
Answer:
Context: These lines are taken from the poem “On His Having Arrived at the Age of Twenty Three”. It was written by the epic poet John Milton. These lines explain the introspection of John Milton.
Explanation : The poet finds displeasure with his development. He attains twenty three years of age. There is no proper development according to the poet. He thinks that God has not provided the talents due to him. He wants to get maturity in writing. But when he comes to introspection, he understands the will and authority of God. Whatever is to be given by God, is given. Hie has a plan for Milton and that will be adopted. Whether the development is slow or fast it is to be accepted by the individual. So, the poet has to wait upon God and get his lot.
General Relevance : Every body in this world has to obey God and his plans. Though there is dissatisfactionin the beginning in course of time the individual will satisfy himself with the blessings of God.
Question 2.
Enough of Science and of Arts,
Close up those barren leaves.
Answer:
Context: These lines are extracted from the poem “The Tables Turned” written by William Wordsworth. These lines suggest how the poet creates interest in the study of nature.
Explanation : These are the lines from the concluding stanza. The central idea of the poem is knowing the importance of nature in education. The poet wants the tables of books to be turned. There is no need of the books. Laborious study is to be shun. There are many books.of science or of arts. They have plenty of knowledge in each subject. But This study is not equivalent with the study of nature which gives a lot of knowledge. Those books do not think of the natural messages available in the nature. So, the poet stresses the need of the study of nature.
General relevance : All the knowledge, including that, which belongs to science and art, is available in the study of nature. So one should not hesitate to go in for the study of nature.

Question 3.
I am their wall against all danger,
Their door against the wind and snow.
Answer:
Context: These are the lines taken from the poem “Any Woman” written by the British writer, Katharine Tynan. It is about the sacred place of a mother in the family.
Explanation: The mother is like a pillar in the family. She takes care of the children every now and then. She gives warmth to the children. She keeps them united. She shows her love and keeps them safe in her lap. She is like a wall when there is a danger for the children. She saves them from the winds and rain. Just like a bird guarding her chicks, the mother saves her children from the outwards dangers. In thfe world she refines her children, to be fit for future life.
General Relevance : A mother is like a mother bird, to the children. Natural calamities create problems but the mother does not leave her children to their fate. She is always at their rescue.
Question 4.
Time is with materials filled;
Our todays and yesterdays,
Are the blocks with which we build.
Answer:
Context: These lines are taken from the poem “The Builders” written by H.W. Longfellow. Here people are compared to builders. The life is the structure and time controls it.
Explanation; The poet aims at constructing the building of life, based on time. There is one experience today and it is like a block for the construction. Today’s block shall become a base for the next day’s construction. The blocks make the building qualitative. So one should look to the blocks and utilise them as time passes. The life of an individual should become great by changing according to time.
General Relevance : Whatever be the experience of today, is like a base for the next day. Time will decide the changes and the life style can be improved, accordingly.
III. Answer ANY TWO of the following questions in about 10-15 lines:(2 × 4 = 8)
Question 1.
What according to bacon is the theme of’of studies’?
Answer:
Francis Bacon was a great english essayist. ‘Of Studies’ is an interesting essay depicting the benefits and disadvantages of studies. , Reading books gives us happiness. It helps us to become more intelligent in communication. Natural abilities can be developed by reading books. Sometimes it becomes harmful if too-much time is spent on it. Showy tendency should be avoided. There are books to be skipped through, books to be read completely and some others to be read with great care and to be digested. Through studies an individual becomes a full man. The author tells that the study of different subjects helps us to cure the weaknesses of the mind.
Question 2.
Explain the views of Huxley about the instruments for experimentation.
Answer:
Aldous Huxley was anEnglish Writer. He wrote a number of books. ‘j.C.Bose’ is an essay taken from the book “Testing Pilate”. It is about the experimenting on Science and Technology. In the beginning of the essay he writes that experimentation does not need expensive instruments. Michael Faraday invented the powers of electricity using very simple things like the tea kettle, silk wires, wax and jam pots only. Similarly J.C.Bose invented the machine, to measure the growth of plant. Here also a little clockwork, needles, filaments, a sheet of smoked glass etc., are used in the experiment. So, the writer thinks that there is no need of costly instruments, if the instrumenter is with curiousity and talent. Even the suffering of a plant or death of a poisoned plant could be seen on the smoked glass. The things are be arranged properly.
Question 3.
What lessons did Dr. Barnard learn from the two children ?
Answer:
Dr. Barnard was a heart surgeon and a writer. He wrote the ” essay “In Celebrations of Being Alive” giving his experience at the time of an accident. There was an incident at the Cape Town Red Cross Children’s Hospital.. The trolly of breakfast was moved by two physically handicapped children. One was blind and the other was a heart patient. They drove it and made it worthy scene for all the patients and nurses around. The boys did not think of their deformities but did whatever they could do with all that they possessed. So, the writer learnt that one should try to utilise the possessions carefully. One should not aspire for that which was not available. The business of living is the celebration of being alive. Thus the poem teaches us the quality satisfaction.

Question 4.
Indians become infinate even without being friendly.
Answer:
N.R.Narayana Murthy was a great Industrialist and Writer. In the essay “Learning from the West” he gives a number of examples from the life of European countries and asks us to emulate. One of the qualities is that the people of the West become friendly even without being intimate. This statement can be seen in the behaviour of Rudyard Kipling. He said that an easterner tries to become intimate without being friendly. In this example we find how an Indian differs from the Western person in behaviour.
IV. Answer ANY TWO of the following questions in about 10-15 lines :(2 × 4 = 8)
Question 1.
‘Time and Tide wait for no man” is an old saying. Discuss this in the context of the poem “On His Having Arrived at the Age Of Twenty Three”.
Answer:
John Milton was an epic poet. He writes in a grand style. “On His Having Arrived at the Age of Twenty Three” is a sonnet, written by this poet. Here the saying of “Time and Tide wait for no man’ is suitable to be discussed. It is a fact that the tides in the sea move on to the shores at a high speed and they cannot be stopped by anything. Similarly time doesnot stay Or stop at any place. It is a natural phenomena. The consumption of time is a continuous process. Milton looks to himself and wonders how his years have passed away. He cannot find a reason in the beginning. So he complains against God.
But after introspection, he finds a solace. He thinks that time does npt wait for him or for his maturity. Thus the saying can be applied to the views of John Milton.
Question 2.
Why does Wordsworth consider nature to be a good teacher ?
Answer:
‘The Tables Turned’ is a famous poem written by William Wordsworth. He was a romantic poet and his love towards nature is of great value. The poet believes that nature is the main source for education. From books, we learn only a little but nature gives us complete knowledge. The beautiful scenery, the lustrous plants and trees, the birds and all that is there give us complete knowledge of the creation. The birds do riot read books but they know the secrets of this world. For research, we dissect and kill certain creatures, But if we have an affectionate heart, we can acquire a lot of knowledge from the nature. So, the poet thinks that the nature is a good teacher.
Question 3.
What is the appropriateness of the title “The Builders” ? Do r you agree with the poet about building one’s own life ?
Answer:
‘The Builders’ is an interesting pqem written by the great American poet H.W Longfellow. The poet compares men to builders. An individual’s life is controlled by time and he is the one who constructs the building of life. According to the poet, everybody plans his life according to his will. He experiences something and advances based on the previous experience. Everyday’s work is taken as a block in the construction of thp building. There are people who build their character on good behaviour, with good blocks. Those lives have become buildings suitable for the dwelling of God. Thus people have to build huge buildings of character. The poet suggests the title, ‘The Builders’ and if is quite apt.
Question 4.
The poem “Any Woman” is a celebration of the glory of womanhood. Illustrate.
Answer:
The poem “Any Woman” was written by Katharine Tynan, a British writer. She produced a number of novels and poems and became a noted writer. Any woman is a poem depicting the supremacy of a woman in the family. She loves her children and guards them carefully. Without a woman, there is no house of recognition. A mother takes care of the children providing food and shelter. She keeps the house neat and tidy. She protects them at the time of danger. If the mother leaves a Child, he will be spoiled. She is like a pillar in the house and keeps the house graceful. A good Woman brings respect to the family.
V. Answer ANY ONE of the following questions in about 25 lines : (1 × 8 = 8)
Question 1.
What are the turning points in the story ? Discuss the main events in the plot.
Answer:
Mark Twain, wrote the novel “The adventures of Tom Sawyer”. The story of Tom Sawyer is narrated by the writer, with interesting turning points at different places- The story moves round the tender boy Tom Sawyer. He was being brought up by Aunt Polly. At the school, there was an interesting episode. He got punishment but he drew a beautiful picture. He showed love towards Becky, a girl in the class. There is,a turning point at the graveyard. The murder of the doctor, by Injun Joe, created frustration in the minds of Tom and Huck. It continued throughout the stoiy until the person died in the cave.
There is an important point, when they attended the funeral function. It adds to the humour of the novel. Muff Potter was jailed but Tom gave witness before the judge and he was set free. Becky’s parents arranged a picnic. Tom and Becky were caught in the cave but were saved. The judge arranged an iron door. This door became the cause for the death of the villain Injiin Joe at the end. Huck was adopted by Widow Dougals. He became a person with culture.
Afterwards Tom wanted to form a pirate gang and Huck would become the bold pirate. Thus, there are some turning points which make the story interesting.

Question 2.
Write at least five lines about the following characters.
a) Auntpolly
b) Muff Potter
c) Becky Thatcher.
Answer:
a) Aunt Polly : Aunt pollywas the care taker of Tom Sawyer. As his mother died, she was carefully looking to the needs of Tom, When she was misguided by Tom, she showed anger but blended with .motherly affection. She wanted the boy to be in a discplined way. When we look to her character, She was kind hearted, cool and considerate. N She was loved by Tom and was deceived at times showing some love towards her. When she saw the boy Tom and his friend Huck at the mourning cermony she was astonished. She was filled with happiness.
She show ther love and affection to both of them. On the whole, the character should have been given based on the life of Twain’s another.
b) Muff Potter : One of the small characters is Muff Potter. He was the father of Huckleberry Finn. He was an aweful drunkard. He ‘ could not provide a place of residence for his son. Muff Potter thought that Injun Joe was his friend. He did not know that he was cheated by him. When the people believed that he was a murderer, his position still degraded. Muff Potter was saved from the plight, when Tom gave . his witness in the. court. He was kind hearted and was not harmful.
But the circumstances drove him into troubles.
c) Becky Thatcher : Becky is an interesting character in the novel. Though she was the beautiful girl at the school along with Tom, she was taken as the heroine. She was at a tenderage and her pretty face was attracted by Tom. The writer did not want to make Tom a hero having maturity. Becky’s love towards Tom was a puppy love. We find her to be sacrificing her life also showing her bravery. Becky was a splendid girl, exhibiting virtues suitable for a tender and pretty girl.
Question 3.
Write a character sketch of Tom Sawyer.
Answer:
Tom Sawyer was the young hero in the novel “The Adventures of Tom Sawyer” written by Mark Twaim, an American writer. Tom was a boy of a tender age. He was a misfit in the school. His behaviour with Becky Thatcher is interesting because he had a liking for her. He expressed it boldy. He was equally like by the girl, Tom had another ” friend, Huck- leberry Finn, who was emtidy, and spent his time fishing and swimming. Tom liked this boy because he wanted to have adventures in life.
Tom went to the graveyard one day along with Huck, to see the ghosts. It was a mysterious experience for him. The three men who came there to rob a dead body quarrelled among themselves. One of them, a doctor was killed by Injun Joe. Tom was frightened to watch Injun killing the doctor. Tom had a liking to wander and his stay at Jackson’s island, was wonderful.
The parents arranged a funeral, thinking that Tom and Huck were dead, which indeed created a humorous occasion for Tom’s jolly mind. Tom’s presence at the church created a hilarious situation. Tom s determination to save Muff Potter and his brave witness at the court, adds to his virtuous behaviour. His relationship with Becky Thatcher was very much appreciative.
Tom wanted to save Widow Bougals. His determination to punish Injun Joe and his pursuasion in the Search of the treasure in the cave, were note worthy. Toni got the treasure and changed Huck, the dirty, lazy and unworthy boy to a neat and tidy boy. Thus Tom was typical of the days of Mark Twainer in America.
SECTION – B
VI. Read the following passage carefully and answer the questions kthat follow: (5 × 1 = 5)
The maneless Lion
Fifty feet away, three male lions lay by the road. They didn’t appear to have a hair on their heads. Noting the colour of their noses (leonine noses darken as they age, from pink to black),
Craig estimated that they were six years old-young adults. “This is what we came to see. They are really maneless.” Craig, a professor at the University of Minnesota, is arguably the leading expert on the majestic Serengeti lion, whose head is mantled, in long, thick hair. He and Peyton West, a doctoral student who has been working with him in Tanzania, had never seen the Tsavo lions that live some 200 miles east of Serengeti. The scientists had partly suspected that the maneless male lions were adolescents mistaken for adults by amateur observers. Now they know better.
The Tsavo research expedition was mostly Peyton’s show. She had spent several years in Tanzania, compiling the data she needed to answer a question that ought to have been answered long ago : Why do lions have manes ? It’s the only cat wild or domestic, that displays such ornamentation.
Questions:
1.Name the two scientists in the passage.
Answer:
Craig and Peyton West.
2.State true or false :
Serengiti lions are maneless.
Answer:
False.
3. Where do Tsavo lions live ?
Answer:
Some 200 miles away from Serengeti.
4.Pick the adjective in the passage which means ‘pertaining to or characteristic of a lion’.
Answer:
Leonine.
5.Write the noun form of the verb ‘estimated’.
Answer:
Estimation.

VII. Read the passage and answer the questions that follow : (5 × 1 = 5)
‘I’m going swimming but you can’t come with me. You’re working,’ said Ben. ‘Do you calllhis work ?’ asked Tom. ‘Of course it’s work. You’re painting a fence,’ said Ben. May be it’s work but may be it isn’t. I like it!’• said Tom, ‘I can swim everyday, but I can’t paint a fence everyday.’
Ben watched Tom. He painted slowly and carefully. He often stopped to and moved back from the fence. He looked at his work and smiled. Ben was suddenly interested in the fence and said, ‘Let me paint a little, Tom.’ Tom thought for a moment. Tm sorry, Ben, Aunt Polly wants me to do it because I’m very good at painting. My brother Sid wanted to do it, but he’s not good at painting.’ ‘Oh please, Tom! Please can I paint ? I’m good at painting too. Here, you can have some of my apple.’ ‘No, Ben I can’t’ – ‘Then take all of my apple !’
Questions :
1.What is the work Tom was doing ?
Answer:
Painting the fence.
2.Was Tom really enjoying his work ?
Answer:
No.
3.Who was suddenly interested in painting the fence ?
Answer:
Ben.
4.What did Ben offer Tom to give him a chance to paint the fence ?
Answer:
An apple.
5.What do you think of Tom from the above incident ?
a.Tom is hard working.
b.Tom is plain natured.
c.Tom is cunning.
Answer:
c. Tom is cunning.
VIII. Study the advertisement given below and answer the questions that follow : (5 × 1 = 5)

1.Where should we cross the road ?
Answer:
At Zebra crossing.
2.Why should we call the National Consumer Help Line ac-cording to the advertisement ?
Answer:
For any help / clarification.
3.What is the toll free number for the National Consumer Help Line ?
Answer:
1800-11-4000.
4.What should we do before we assert our right ?
Answer:
We should be responsible to follow rules / It’s our responsibility to follow rules.
5.Write the noun form of ‘assert’.
Answer:
Assertion.
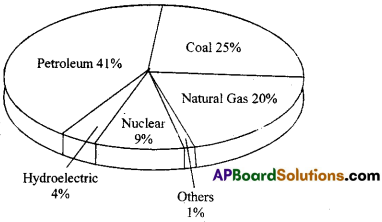
IX. Study the Pie Chart carefully and answer the questions that follow. (5 × 1 = 5)
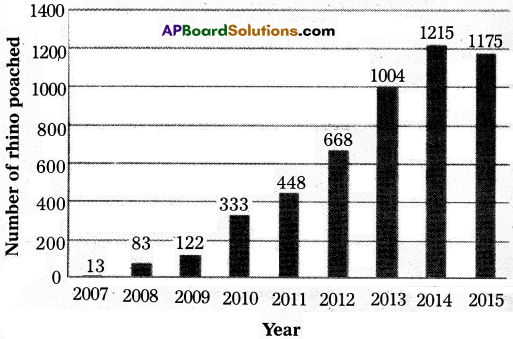
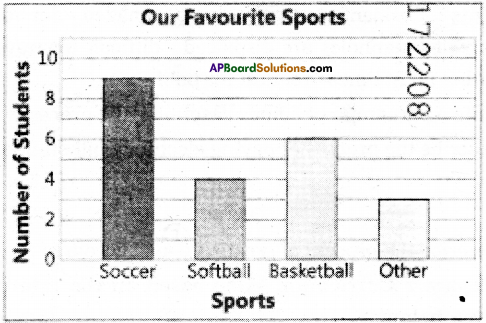
The following bar graph shows the number of rhinoceros poached in South Africa from 2007 to 2015.
Recorded number of rhinos poached in South Africa
Questions :
1.In which year were the maximum rhinos poached ?
Answer:
2014.
2.Explain if poaching decreased or increased from 2007 to 2015.
Answer:
Poaching increased from 2007 to 2014 and decreased a bit in 2015.
3.During which years can we observe the maximum difference in the number of rhinos poached ?
Answer:
2012 to 2013.
4.A person who poaches is called a.
Answer:
Poacher.
5.State true or false.
The number of rhinos poached in 2014 is almost double to the number, poached in 2012..
Answer:
True.

SECTION – C
X. Write a letter to the editor of a newspaper about the bad condition of roads in a locality.(1 × 5 = 5)
You are interested in doing a short term course in Spoken English during your summer vacation. Write a letter to the director, NEO Spoken English and Grammar Institute, Annamaiah circle, Tirupati, enquiring about the terms and conditions for the course.
Answer:
K.Amaranath
2-3-40 Gandhinagar
Vijayawada.
15-4-2019
The Director
NEO Spoken English Institute
Annamalai circle
Tirupathi.
Sir,
Sub: Information about short term course-request-regarding. Having come to know that the institute is running a short term course in spoken English. I wish to know the particulars about it/1 wish to know the duration of the course and the date of starting. The fee particulars, the time of running the classes, may also be sent. Is there any hostel facility ? If there is hostel, the fee there of may also’be informed.
Thanking you
Yours faithfully
K.Amaranath.
OR
You have been invited to attend your friend’s marriage. You are not able to attend the marriage due to personal problems. Write a letter to your friend congratulating him and expressing your inability to attend.his marriage.
Answer:
10-25, Srinagar colony
Guntur.
10-6-2019
My dear Ramana,
I am doing well and hope the same with you. Here is a piece of obligation to you. First of all accept my hearty congratulations, for the happy occasion of your wedding recently. At the same time I request you to pardon me, for I could not be there at that time. I had an interview in Delhi two days before the marriage.
I could not reach Vijayawada by that date. Hope you would, understand my inability. Convey my best wishes to your better half.
With Love
Yours lovingly
D. Ravindra
Address:
B.VRamana
2-3-11.0 Narasimhanagar
Vishakhapatnam.

XI. Write a short paragraph describing the process of how you get ready for college regularly.(1 × 5 = 5)
Answer:
These days study to a college, needs so much care and preparation. Everyday I have to attend my college at 8 A.M’. So, I get up at 6 A.M and finish off the daily chores in time. I should not be lazy in getting up from bed. I will have an eye upon anytime table, to sort out the books necessary. I have to verify whether all the Home work given has been finished or not. As, I go to college by bus, I must start from my house early. I will take the breakfast, sufficiently early. My mother keeps the box of meals ready. 1 will take water in a bottle. The bag of books is kept ready and so, I go to the main road taking the bag, the bottle and others. I wait there until the college bus arrives the point of halt. Then I reach the college and go on with the classwork. It is a routine work.
OR
Describe the process of making tea for two people.
Answer:
It would be better if’we learn certain things, needed for our smooth living. How to make tea for two people. The following are needed to prepare tea.
Tea Kettle
Spoons
Filter
Cups and Saucers
Tea powder
Sugar
Milk.
First of all take some water in the kettle. Mix some tea powder in it and boil it for a few minutes. Pour some milk into it and boil it for another few minutes. Take the kettle out of the stove and filter the tea. Add some sugar into the tea. Serve the tea in two cups to the people waiting.
XII.Prepare a curriculum vitae in response to the following advertisement.(1 × 5 = 5)

Now prepare a Resume based on the above format. In this Resume, Dandi Deepa is applying for the position of Area Sales Manager in the reputed NCL Company based on the newspaper advertisement given at the beginning of this section.
Answer:
DANDI DEEPA
45/24, Teachers Colony
Venkata Ramana Colony Road No. 4
KURNOOL – 518003
Mobile : 8500012340
kdeepa@gmail.com
OBJECTIVE
A position as Area Sales Manager in a reputed company.
EDUCATION
2009-2011 – MBA (Sales & Marketing)
Sri Padmavati Mahila Visvavidyalayam, Tirupati, 81% aggregate.
2006-2009 – B.Com (General) Silver Jubilee College (Autonomous), Kumool, 76% aggregate.
2004-2006 – Intermediate (C.E.C), Board of Intermediate Education, AP. Govt. Junior College (Town), Kumool, 82.5% aggregate.
2004 – SSC, Board of Secondary Education, AE Govt. Girls High School, Kumool, 80% aggregate.
WORK EXPERIENCE
2011-2016 Worked as Sales Officer for five years in XYZ Company, Vijayawada.
2016 – Present Working as Area Sales Manager for three years in Abhiram Industries, Ananthapuram.
STRENGTHS
Good communication skills, Problem solving and Managerial skills, Good motivator and coordinator.
ADDITIONAL INFORMATION
Languages known : English, Telugu and Hindi
Hobbies : Reading books and listening to music
Father’s Name : Sri Dandi Gopal Rao
References : Available on request
DECLARATION
I hereby declare that the details furnished above are true and correct to the best of my knowledge and belief and I undertake to inform you of any changes therein, immediately.
D. Deepa
SIGNATURE
Place : Kumool.
Date : 23 April, 2019.
A cover, letter which is to be sent with the CV/Resume of Dandi Deepa is given below.
DANDI DEEPA
45/24, Teachers Colony
Venkata Ramana Colony Road No. 4
KURNOOL – 518003
Mobile : 8500012340
23 April, 2019
kdeepa@gmail.com.
The Manager
M/s NCL Industries Ltd.,
Gayathri Nursing Home, Near BSNL Tel. Exchange
TUDA Plots, RC Road, Tirupati-517501
Dear Sir
APPLICATION FOR AREA SALES MANAGER
In response to your advertisement in the Eenadu newspaper of April 23, 2019 for the post of Area Sales Manager, I am enclosing my resume for your consideration. I feel I have the required qualifications and skills for the position in your industry.
I completed my academics with good percentage. Moreover, my work experience in the area you have asked for is very effective and always I worked for the benefit of the companies and proved myself worthy in the positions I held.
I am excited to see your job listed in the newspaper. I have been looking for exactly this position, and I think that my work experience and related skills will be an excellent match for your needs.
You can call me for an interview on any day convenient to you. With best regards
Yours faithfully
D. Deepa .
(DANDI DEEPA)
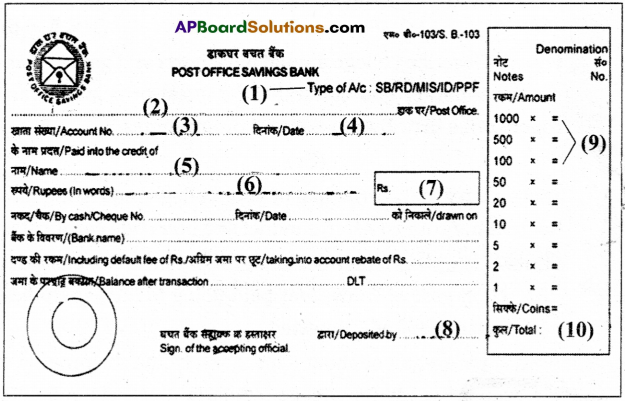
XIII.Fill in the form (10 × 1/2 = 5)
Mr. Rajesh Kumar wants to subscribe to the Textile Magazine for a period of two years. He is enclosing a Cheque for the mentioned amount bearing No. 244261 dated 12.06.2019. He lives at H.No. 124/2-B, Prakash Nagar, Narasaraopet. – 522601. His rpobile No. is 944164901 and his Mail Id is rkumar1995@gmail.com.

Answer:
1) The Textile Magazine 2 years (24 issues) ? 800
2) Mr. Rajesh Kumar
3) Personal
4) No
5) H.No. 124/2-B Prakash Nagar, Narasaraopet
6) 944164901 .
7) rkumar1995@gmail.com
8) 244261
9) 12-06-2019
10) 800

XIV.Construct adialogue between the doctor and the patient about treatment for fever and pain. (1 × 5 = 5)
Answer:
Patient : Good morning, doctor.
Doctor : Good morning, What’s your problem ?
Patient : Sir, I’m feeling weak and I’m unable to walk even a 5 little distance.
Doctor : What’s your appetite like ?
Patient : . Not at all good. I don’t feel like eating anything.
Doctor : Have you had any fever ?
Patient : Well, I do feel feverish all the time.
Doctor : All right, let me check your temperature first. There’s nothing wrong with the pulse. You please lie down on that table, I will examine.
Patient : Yes, doctor.
Doctor : Do you feel pain here ?
Patient : Yes, some.
Doctor : And here ?
Patient : Oh! That’s quite painful.
Doctor : Nothing to worry. I’m prescribing two types of tablets. Take one before meals and the other after meals ‘ – for three days. Don’t eat any fried or spicy food. Drink
milk and follow your regular diet.
Patient : Thank you doctor.
Doctor : It’s ok.
OR
Write a dialogue between a customer and the waiter of a hotel (enquire about room for two days)
Answer:
Customer : Waiter!
Waiter : Yes sir, what can I do for you ?
Customer : Bring the Menu card.
Waiter : Sir, here it is.
Customer : Is this veg or non-veg ?
Waiter : It is purely veg sir.
Customer : OK, what do you have, hot ?
Waiter : Idly, vada and dosa are hot.
Customer : Bring me a plate of Idly and tell the master to prepare a dosa also.
Waiter : Yes sir.
Customer : Waiter!
Waiter : Yes, please.
Customer : This jug is empty. Pour some water. Is it aqua water ?
Waiter : Yes sir.
Customer : Where’s the wash basin ?
Waiter : That side, sir.
Customer : Waiter! Get me a cup of coffee.
Waiter : Here you are, sir.
Customer : Get me the bill.
Waiter : Yes sir, here’s the change.
Customer : Keep it for you.
Waiter : Thank you sir.

XV.Read the following and make notes. (1 × 5 = 5)
Women empowerment has becorpe the buzzword today with women working alongside men in all spheres. They profess an independent outlook, whether they are living inside their home or working outside. They are increasingly gaining control over their lives and taking their own decisions with regard to their education, career, profession and lifestyle.
With steady increase in the number of working women, they have gained financial independence, which has given them con-fidence to lead their own lives and build their own identity. They are successfully taking up diverse professions to prove that they are second to none is any respect.
But while doing so, women also take care to strike a balance between their commitment to their profession as well as their home and family. They are playing multiple roles of a mother, daughter, sister, wife and a working professional with remarkable harmony and ease. With equal opportunities to work, they are functioning with a spirit of team work to render all possible co-operation to their male counter parts in meeting the deadlines and targets set in their respective professions!
Women empowerment is not limited to urban, working women but women in even remote towns and villages are now increasingly making their voices heard loud and clear in society. They are no longer willing to play a second fiddle to their male counter parts. Educated or not, they are asserting their social and political rights and making their presence felt, regardless pf their socioeconomic backgrounds.
While it is true that women, by and large, do not face discrimi-nation in society today, unfortunately, many of them face exploita-tion and harassment which can be of diverse types : emotional, physical, mental and sexual. They are often subjected to rape, abuse and other forms of physical and intellectual violence.
Women empowerment, in the trust sense, will be achieved only when there is attitudinal change in society with regard to womenfolk, treating them with proper respect, dignity, fairness and equality. The rural areas of the country are, by and large, steeped in a feudal and medieval outlook, refusing to grant women equal say in the matters of their education, marriage, dress code, profession and social interactions.
Let us hope, women empowerment spreads to progressive as well as backward areas of our vast country.
Answer:
Women empowerment : Now-a-days women are trying to be independent-own decisions – Financial independence for women – second to none in every respect.
Woman in society : Mother, Daughter, Sister-tearti,work-co-operation from male partners – respect in professions.
Place: Both urban and rural areas to develop – social and political rights – No exploitation of ladies.
Prosperity : Women to prosper – respect, dignity and equality wrought.
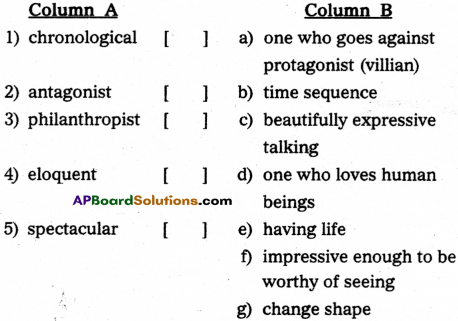
XVI.Match the wards in Column A with their meanings/definitions in Column ‘B’.
| Column ‘A’ |
Column ‘B’ |
| 1.incredible |
a.call on for inspiration |
| 2.contradict |
b.throwout |
| 3.invoke |
c.say against |
| 4.eject |
d.a system where there is no rule |
| 5.anarchy |
e.that which cannot be believe unbelievable |
|
f.shaped like a cross |
|
g.mathematical shape |
Answer:
| Column A |
Column ‘B’ |
| 1. interact |
e.that which cannot be believe unbelievable |
| 2. animate. |
c.say against |
| 3. calligraphy |
a.call on for inspiration |
| 4. cacophony |
b.throwout |
| 5. egoity |
d.a system where there is no rule |

XVII. Mark the stress for ANY FIVE of the following words : (5 × 1 = 5)
1) minister
2) parliament
3)nation
4) confidential
5)kilometer
6) tourism
7)commandeer
8) telegraph
9) ornament
10) architect
Answer:
1) minister = ‘minister
2) parliament = ‘parliament
3) nation = ‘nation
4) confidential = confi’dential
5) kilometer = ‘kilometer
6), tourisom = ‘tourisom
7) commander = comman’der
8) telegraph = te’legraph
9) ornament = ‘ornament
10) architect = ‘architect
![]()
![]()

![]()

![]()
![]()
![]()














 Questions :
Questions :