Access to a variety of TS Inter 2nd Year Chemistry Model Papers and TS Inter 2nd Year Chemistry Question Paper March 2018 helps students overcome exam anxiety by fostering familiarity.
TS Inter 2nd Year Chemistry Question Paper March 2018
Note : Read the following instructions carefully.
- Answer all questions of Section – ‘A’. Answer any six questions in Section – ‘B’ and any two questions in Section – ‘C’.
- In Section – A, questions from Sr. Nos. 1 to 10 are of “Very Short Answer Type”. Each question carries two marks. Every answer may be limited to 2 or 3 sentences. Answer all these questions at one place in the same order.
- In Section -”’8′, questions from Sr. Nos. 11 to 18 are of “Short Answer Type”. Each question carries four marks. Every answer may be limited to 75 words.
- In Section – ‘C’, questions from Sr. Nos. 19 to 21 are of “Long Answer Type”. Each question carries eight marks. Every answer may be limited to 300 words.
- Draw labelled diagrams wherever necessary for questions in Section – ‘B’ and ‘C’.
Section – A
Note : Answer all the questions.
Question 1.
State Raoult’s law.
Answer:
Raoult’s law for non-volatile solute : The relative lowering of vapour pressure of dilute solution containing non-volatile solute is equal to the mole fraction of solute. 1
Question 2.
State Faraday’s second law of electrolysis.
Answer:
The amounts of different substances liberated when the same quantity of electricity is passed through the electrolytic solution are proportional to their chemical equivalent weights.
![]()
Question 3.
What is PHBV? How is it useful to man?
Answer:
Poly p – hydroxy butyrate – CO – P – hydroxy Valerate (PHBV) : It is a Copolymer of 3 -hydroxy butanoic acid and 3 – hydroxy pentanoic acid.
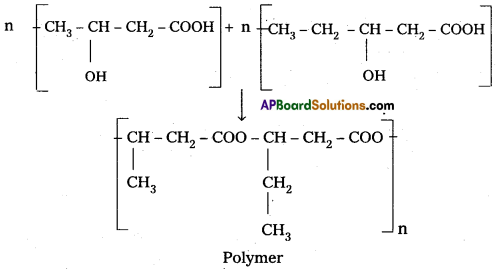
It is used in medicine for making capsules. PHBV also undergoes degradation by bacteria.
Question 4.
Write the names of the monomers for the following polymers.
a) Bakelite
b) Nylon 6, 6
Answer:
a) Bakelite : In Bakelite monomers are phenol.
(C6H5OH) and formaldehyde (HCHO)
Monomers : Phenol, Formaldehyde
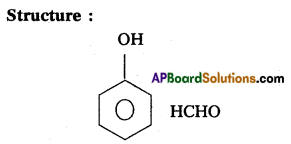
b) Nylon 6, 6 :
The repeating monomeric units of Nylon 6, 6 are hexamethylene diamine and Adipic acid.

Question 5.
What is a ligand ? Give one example for unidentate ligand.
Answer:
Ligand : A co-ordinating entity which is bound to the central atom by donating electron pairs is called a ligand.
Eg. : Cl–, NH3, CN– etc.
A unidentate ligand containing two possible donor atoms can co-ordinate through either of donor atoms. Such ligands are called ambidentate ligands.
Eg : NO2–
![]()
Question 6.
What are the neutral oxides of nitrogen?
Answer:
Nitrous oxide (N2O) and Nitric oxide (NO) are neutral oxides of nitrogen.
Question 7.
What happens when Cl2 reacts with dry slaked lime?
Answer:
Chlorine reacts with dry slaked lime and forms bleaching powder.
Ca(OH)2 + Cl2 → CaOCl2 + H2O
Question 8.
What is blister copper? Why is it so called?
Answer:
During the extraction of ‘Cu’ from copper pyrites when the matte is charged into a Bessemer converter then cuprous oxide combine with cuprous sulphide and forms Cu metal.
2CU2o + Cu2S → 6Cu + SO2 The ‘Cu’ metal is cooled.
The obtained copper metal is impure and is known as “Blister copper” (98% pure). The solid fied copper has blistered appearance due to evolution of SOz Hence it is called blister copper.
Question 9.
Explain carbylatnine reaction with an example.
Answer:
Aniline reacts with chloroform in presence of ale. KOH to form phenyl isocyanide.
C6H5 NH2 + CHCl3 + 3KOH → C6H5 NC + 3KCl + 3H2O
Question 10.
Explain the reaction of Aniline with Nitrous acid.
Answer:
Reaction of Aniline with nitrous acid : Aniline reacts with nitrous acid at low temperatures (0 – 5°C) to form diazonium salts. (Benzene diazonium salt)
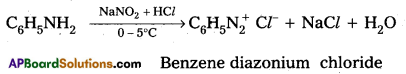
Section – B
Question 11.
Explain Schottky and Frenkel defects.
Answer:
Schottky defect :
- It is a point defect in which an atom or ion is missing from its normal site in the lattice”.
- In order to maintain electrical neutrality, the number of missing cations and anions are equal.
- This sort of defect occurs mainly in highly ionic compounds, where cationic and anionic sizes are similar. In such compounds the co-ordination number in high. Ex. : NaCl, CsCl etc.
- Illustration :
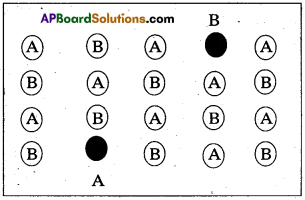
- This defect decreases the density of the substance.
Frenkel defect :
- “It is a point defect in which an atom or ion is shifted from its normal lattice position”. The ion or the atom now occupies an interstitial position in the lattice.
- This type of a defect is favoured by a large difference in sizes between the cation and anion. In these compounds co-ordination number is low. E.g.: Ag – halides, ZnS etc.
- Illustration :
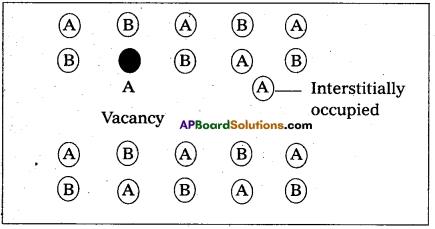
- Frenkel defect do not change the density of the solids significantly.
Question 12.
Define mole fraction. Calculate the mole fraction of H2SO4 in a solution containing 98% (w/w) H2SO4 by mass.
Answer:
Mole fraction : The ratio of number of moles of the one component of the solution to the total number of moles of all the components of the solution is called mole fraction.
Mole fraction of solute Xs = \(\frac{n_s}{n_0+n_s}\)
Mole fraction of solvent X0 = \(\frac{n_s}{n_0+n_s}\)
ns = number of moles of solute
n0 = number of moles of solvent
→ It has no units.
Given a solution containing – 98% H2SO4 by mass. It means 98 gms of H2SO4 and 2 gms of H2O mixed to form a solution.
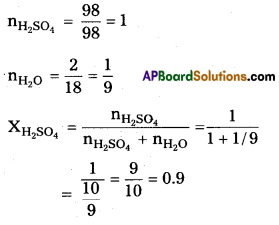
Question 13.
What are ’emulsions? How are they classified ? Give one example for each.
Answer:
Emulsion: The colloidal system in which a dispersion of finely divided droplets of a liquid in another liquid medium is called Emulsion. Ex : Milk.
In Milk, the droplets of liquid fat are dispersed in water. This is an example for oil in water type emulsion.
Classification of emulsions : Emulsions are classified into two classes. These are
a) Oil in Water (O/W) and
b) Water in Oil (W/O), (O = Oil; W = Water).
These emulsions are classified as such depending on which is dispersed phase and which is dispersion medium.
a) Oil in Water (O/W) type emulsions:
In this type of emulsions, the dispersed phase is oil and the dispersion medium is water.
Ex : Milk, liquid, fat (oil) in water. Vanishing cream; fat in water.
b) Water in Oil (W/O) type emulsions:
In this type of emulsions the dispersed phase is water and the dispersion medium is oil.
Ex : Stiff greases : water in lubrication oils
Cod liver oil : water in cod liver oil
Cold cream : water in fat.
![]()
Question 14.
Explain calcination and roasting with suitable examples.
Answer:
Roasting : Removal of the volatile components of a mineral by heating mineral either alone (or) mixed with some other substances to a high temperature in the presence of air is called Roasting.
- It is applied to the sulphide ores. .
- SO2 gas is producted along with metal oxide.
![]()
Calcination :
Removal of the volatile components of a mineral by heating in the absence of air is called calcination.
- It is applied to carbonates and bicarbonates.
- CO2 gas is produced along with metal oxide.
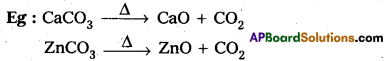
Question 15.
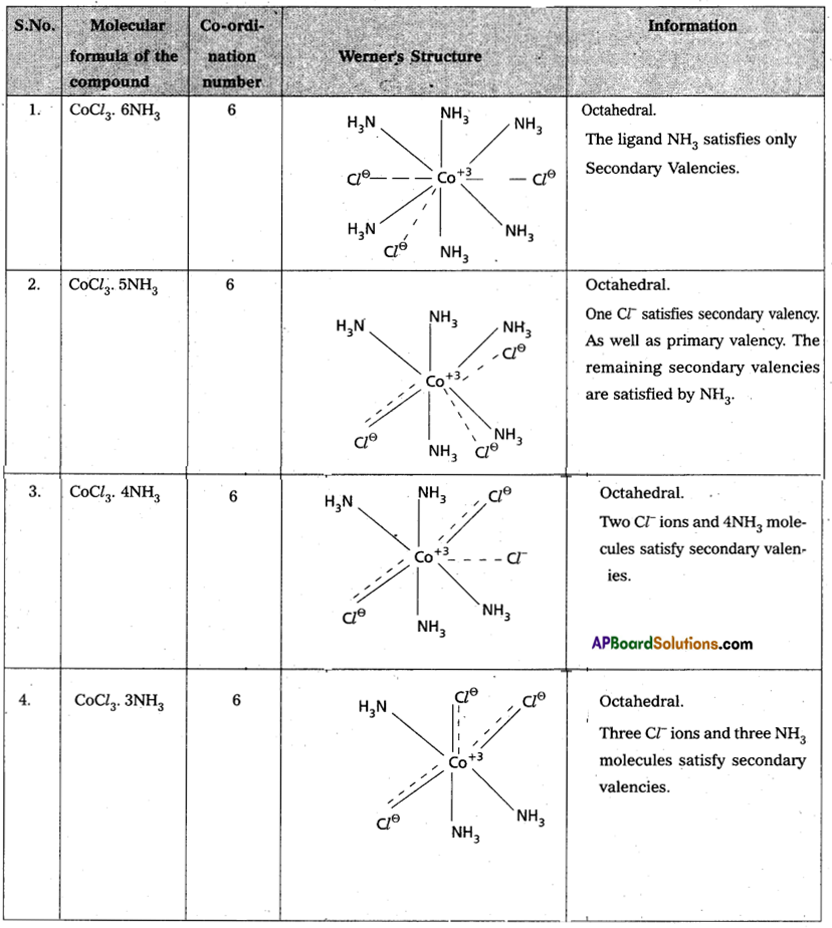
Explain Werner’s theory of coordination compounds with suitable examples.
Answer:
Werner’s theory :
Postulates :
- Every complex compound has a central metal atom (or) s ion.
- The central metal shows two types of valencies namely primary valency and secondary valency.
A) Primary valency:
The primary valency is numerically equal to the oxidation state of the metal. Species or groups bound by primary valencies undergo complete ionization. These valencies are identical with ionic bonds and are non-directional. These valencies are represented by discontinuous lines (……)
Eg. : C0Cl3 contains CO3+ and 3Cl– ions. There are three Primary Valencies or three ionic bonds.
B) Secondary Valency:
Each metal has a characteristic number of Secondary Valencies. They are directed in space around the central metal. The number of Secondary Valencies is called Coordination number (C.N.) of the metal. These valencies are directional in Nature.For example in COCl3 6NH3
Three Cl– ions are held by primary Valencies and 6NH3 molecules are held by Secondary Valencies. In CuSO4. 4NH3 complex SO42- ion is held by two Primary Valencies and 4NH3 molecules are held by Secondary Valencies.
3) Some negative ligands, depending upon the complex, may satisfy both primary and secondary valencies. Such ligands, in a complex, which satisfy both primary as well as secondary valencies do not ionize.
4) The primary valency of a metal is known as its outer sphere of attraction or ionizable valency while the Secondary Valencies are known as the inner sphere of attraction or coordination sphere. Groups bound by secondary valencies do not undergo ionization in the complex.
Question 16.
What are hormones? Give one example for eaph of the following.
a) Steroid hormones.
b) Polypeptide hormones.
c) Amino acid derivations.
Answer:
Hormones : Hormone is defined as an “organic compound synthesised by the ductless glands of the body and carried by the blood stream to another part of the body for its function”.
Eg : testosterone, estrogen.
- Example for steroid hormones : Testosterone, Estrogen.
- Example for poly peptide hormones : Insulin
- Example for Amino acid derivative: Thyroidal hormones thyroxine.
![]()
Question 17.
What are analgesics ? How are they classified? Give one example for each.
Answer:
Analgesics: These are to, reduce or totally abolish pain without. ‘causing impairment of consciousness, mental confusion, incoordination, paralysis, disturbances of nervous system etc.
Analgesics are classified as
i) Narcotic analgesics :
These are most potent and clinically useful agents causing depression of central nervous system and at the same time act as strong analgesics. E.g. : Morphine, Codeine etc.
ii) Non-narcotic analgesics : These drugs are analgesics but they have no addictive properties. Their analgesic use is limited to mild aches and pains. E.g. : Aspirin, Ibuprofen etc.
Question 18.
Explain and reactions with an example.
Answer:
i) SN1 : Substitution nucleophilic unimolecular reaction :
In this reaction the first step is the formation of stable carbonium ion. This step is slow and is the rate determining step. The alkyl halides which form stable carbonium ion follow SN1 reaction.
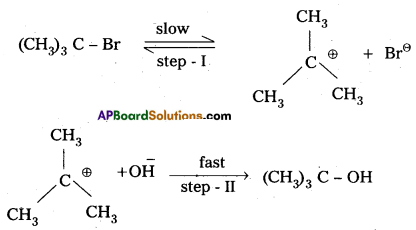
The order of reactivity of alkyl halides towards SN1 reaction.
Tertiary halide > Secondary halide > Primary halide > CH3 – X.
Benzyl halide and allyl halides are primary halides but participate in SN1 mechanism due to the formation of stable benzyl and allyl carbonium ions (Benzyl and allyl carbonium ions are stabilised due to resonance).
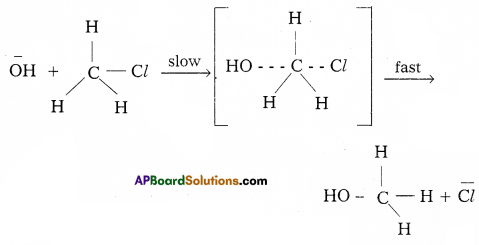
ii) SN2 : Substitution nucleophilic bimolecular reaction :
In this reaction the rate of reaction depends on the concentration of alkyl halide and also on the concentration of nucleophile hence it is a bimolecular reaction.
The order of reactivity of alkyl halides towards SN2 reaction.
CH3 – X > Primary halide > Secondary halide > Tertiary halide For a given alkyl group, the reaction of the alkyl halide, R – X follows the same’ order in both the mechanisms R-I > R – Br > R – Cl > R – F
Question 19.
a) What are Galvanic cells? Explain the working of a Galvanic cell by taking Daniel cell as example.
Answer:
Galvanic cell : A device which converts chemical energy into electrical energy by the use of spontaneous redox reaction is called Galvanic cell (dr) voltaic cell.
Eg : Daniel cell.
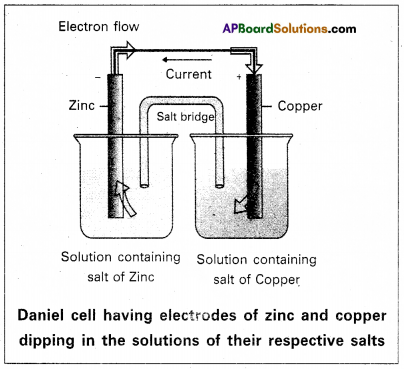
Daniel cell: It is a special type of galvanic cell. It contains two half cells in the same vessel.
The vessel is devided into two chambers. Left chamber is filled with ZnSO4 (aq) solution and Zn – rod is dipped into it. Right chamber is filled with aq. CuSO2 solution and a copper rod is dipped into it. Process diaphragm acts as Salt bridge. The two half cell’s are connected to external battery.
Cell reactions : Ion Zn/ZnSO4 half cell, oxidation, reaction occurs
Zn → Zn2+ + 2e–
Ion Cu/CuSO4 half cell, reduction reaction occurs.
Cu+2 + 2e– → Cu
The net cell reaction is
Zn + Cu+2 ⇌ Zn+2 + Cu
Cell is represented as Zn / Zn+2 || Cu2+ / Cu
b) Describe the salient features of the collision theory of reaction rates.
Answer:
Collision theory of reaction rate bimolecular reactions salient features :
- The reaction molecules are assumed to be hard spheres.
- The reaction is postulated to occur when molecules collide with each other.
- The number of collisions per second per unit volume of the reaction mixture is known as collision frequency (Z).
- For a bimolecular elementary reaction A + B —> products
K = ZAB. eEa/RT ; ZAB = Collision frequency. - All collisions, do not lead to product formation.
- The collisions with sufficient kinetic energy (Threshold energy) are responsible for product formation. These are called as effective collisions.
- To account for effective collisions a factor p called to probability factor or steric factor is introduced.
K = P ZAB. eEa/RT
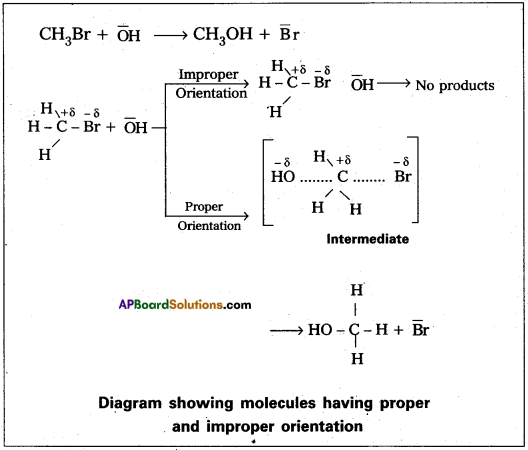
Question 20.
a) Write the important reactions involved in the manufacture of sulphuric acid by ‘contact process’.
Answer:
Manufacture of H2SO4.by contact process : Manufacturing of H2SO4 involves three main steps.
Step-1 :
SO2 production : The required SO2 for this process is obtained by burning S (or) Iron pyrites in oxygen.
S + O2 → SO2
4FeS2 + 15O2 > 2Fe2O3 + 8SO3
Step-2 :
SO3 formation : SO2 is oxidised in presence of catalyst with atmosphric air to form SO3.
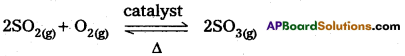
The equation reveals the following points :
- 3 volumes of the reactants convert into 2 volumes of SO3. i.e., a decrease of volume accompanies the reaction.
- The reaction is an exothermic change.
- The catalyst may be present to increase the SO3 yields.
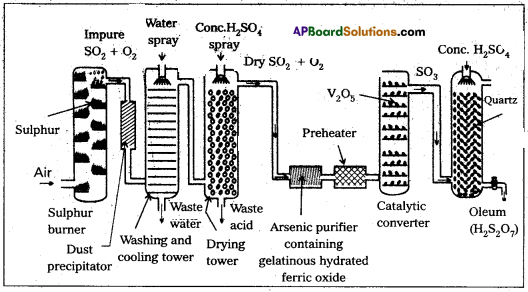
Step-3 :
Formation of H2SO4: SO3 formed in the above step absorbed in 98% H2SO4 to get oleum (H2S2O7). This oleum is diluted to get H2SO4
SO3 + H2SO4 → H2S2O7
H2S2O7 + H2O → 2H2SO4
b) Write the preparation of the compounds XeF2 and XeF4. Give their structures.
Answer:
Xenon forms the binary fluorides XeF2, XeF4, XeF6 as follows. These are formed by direct combination of Xe and F2.
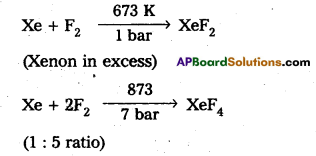
structures of XeF2 :
1) In XeF2 central atom is ‘Xe’
2) ‘Xe’ undergoes sp3d hybridisation in it’s 1st excited state
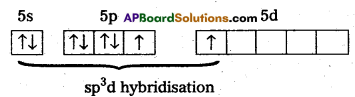
3) Shape of molecule is linear.
4) Xe form two σ.bonds with two fluorines.
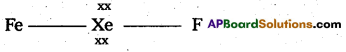
b) structures of XeF4:
1) Central atom in XeF4 is ‘Xe’.
2) Xe undergoes sp3d2 hybridisation in it’s 2nd excited, state.
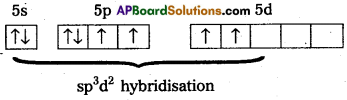
3) Shape of the molecule is square planar with bond angle 90° and bond length 1.95A.
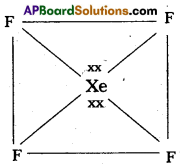
4) Xe – forms four o-bonds by the overlap of sp3d2 – 2pZ (F) orbitals.
Question 21.
Explain the following reactions with equations.
i) Kolbe’s reaction.
ii) Riemer – Tiemann reaction,
iii) Williamson’s ether synthesis,
iv) HVZ reaction.
Answer:
i) Kolbe’s reaction :
Phenol reacts with sodium hydroxide to form sodium phenoxide. This undergoes electrophillic substitution with CO2 to form salicylic acid.
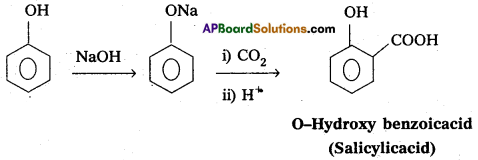
ii) Reimer-Tiemann reaction :
Phenol reacts with chloroform in presence of NaOH to form salicylaldehyde (O-Hydroxy benzal- dehyde). This reaction is known as ii) Reimer-Tiemann reaction : Phenol reacts with chloroform in presence of NaOH to form salicylaldehyde (O-Hydroxy benzal- dehyde). This reaction is known as Reimer-Tiemann reaction.
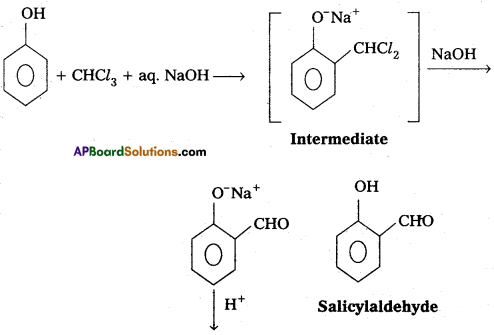
iii) Williamsons ether synthesis :
- This method is used for the preparation of symmetrical and unsymmetrical ethers.
- The reaction of an alkyl halide with sodium alkoxide to form ethers is known as Williamson’s Synthesis.
![]()
E.g. C2H5Cl + CH3ONa → CH3 – O – CH2CH3 + NaCl
(CH3)3 – C -ONa + CH3 – Br → CH3 – O – C – (CH3)3 + NaBr
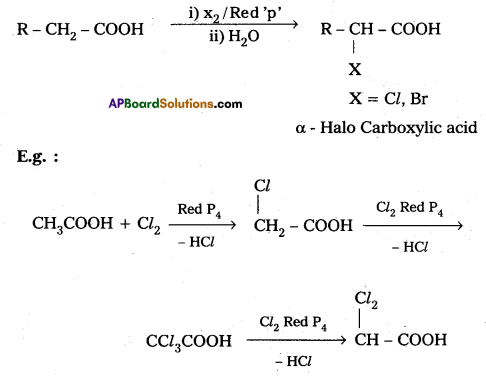
iv) HVZ reaction :
Carboxylic acids having α-hydrogens are halogenated at the α-position on treatment with chlorine or bromine in presence of small amount of red phosphorus to give α-halo carbo¬xylic acids. This reaction is named as Hell-Volhard – Zelinsky (HVZ) reactions.