Access to a variety of TS Inter 2nd Year Chemistry Model Papers and TS Inter 2nd Year Chemistry Question Paper May 2017 helps students overcome exam anxiety by fostering familiarity.
TS Inter 2nd Year Chemistry Question Paper May 2017
Note : Read the following instructions carefully.
- Answer all questions of Section – ‘A’. Answer any six questions in Section – ‘B’ and any two questions in Section – ‘C’.
- In Section – A, questions from Sr. Nos. 1 to 10 are of “Very Short Answer Type”. Each question carries two marks. Every answer may be limited to 2 or 3 sentences. Answer all these questions at one place in the same order.
- In Section -”’8′, questions from Sr. Nos. 11 to 18 are of “Short Answer Type”. Each question carries four marks. Every answer may be limited to 75 words.
- In Section – ‘C’, questions from Sr. Nos. 19 to 21 are of “Long Answer Type”. Each question carries eight marks. Every answer may be limited to 300 words.
- Draw labelled diagrams wherever necessary for questions in Section – ‘B’ and ‘C’.
Section – A
Note : Answer all the questions.
Question 1.
What is ideal solution?
Answer:
The solution which obeys Raoult’s law over the entire range of concentration is known as ideal solution.
Question 2.
Give two examples of zero order reactions.
Answer:
Examples for zero order reactions :
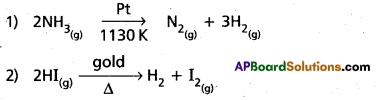
Question 3.
Write any two ores with formulas of the following metals.
(a) Iron
(b) Copper
Answer:
a) Ores of Iron :
Haematite (Fe2O3)
Magnetite (Fe3O4)
b) Ores of Copper:
Copper pyrites (CuFeS2)
Copper glance (Cu2S)
![]()
Question 4.
What is tailing of mercury? How is it removed?
Question 5.
Noble gases are inert. Explain.
Answer:
Noble gases are chemically inert:
1) Noble gases have stable electronic configuration octet configuration except He.
2) Noble gases have high Ionisation energy values and have large positive values of electron gain enthalpy.
Question 6.
Why Zn+2 is diamagnetic where as Mn+2 is paramagnetic?
Question 7.
What is zwitter iron 7 Give anexample.
Answer:
Zwitter ion : In aqueous solution of amino acids, the carboxyl group can lose a proton and amino acid can accept that proton to form a dipolar ion. This ion is called as zwitter ion.
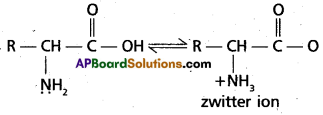
Question 8.
Write any two biological functions of Nucleic acids.
Answer:
Biological functions of Nucleic acids:
- DNA is the chemical basis of heredity.
- DNA is the reserve of genetic information.
- DNA is capable of self duplication during cell division and identical DNA strands are transfered to daughter cells.
- RNA molecules are used in the protein synthesis in the cell.
- The message for the synthesis of a protein is present in DNA.
Question 9.
What are antibiotics? Give an example.
Answer:
Antibiotics : The chemical substances produced by micro organisms and inhibit the growth or destroy micro-organisms are called antibiotics.
(or)
The substance produced totally or partly by chemical synthesis which in low concentration inhibits the growth (or) destroy micro-organism by intervening in their metabolic process are called antibiotics. E.g. : Penicillin, Chloramphenicol etc.
![]()
Question 10.
What are antifertility drugs? Give an example.
Answer:
Antifertility drugs : These are birth control pills and contain a mixture of synthetic oestrogen and progesterone derivatives. E.g. : Norethindrone, Ethynylestradiol (novestrol).
Section – B
Question 11.
What is relative lowering of vapour pressure ? How is it useful to determine the molar mass of a solute?
Answer:
Raoult’s law for volatile solute:
For a solution of volatile liquids,the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution.
Raoult’s law for non-volatile solute :
The relative lowering of vapour pressure of dilute solution containing non-volatile solute is equal
to the mole fraction of solute.
Relative lowering of vapour pressure \(\frac{P_0-P_5}{P_0}\) = XS (mole fraction of solute)
\(\frac{P_0-P_s^{\prime}}{P_0}\) = \(\frac{n_s}{n_0+n_s}\)
For very much dilute solutions ns <<< …..n0
∴ \(\frac{P_0-P_5}{P_0}=\frac{n_5}{n_0}=\frac{w}{m} \times \frac{M}{W}\)
W – Weight of solute
m = Molar mass of solute
w = Weight of solvent
M = Molar mass of solvent
Molar mass of solute m = \(\frac{w \times M}{W} \times \frac{P_0}{P_0-P_s}\)
Question 12.
Describe the two main types of semiconductors and contrast their conduction mechanism.
Answer:
The solids which are^havfng~Thoderate conductivity between insulators and conductors are called semi conductors.
- These have the conductivity range from 10-6 to 104 – Ohm-1m-1.
- By doping process the conductivity of semi conductors increases. E.g. : Si, Ge, crystal.
Semi conductors are of two types. They are :
1. Intrinsic semi-conductors:
In case of semi-conductors, the gap between the valence band and conduction band is small. Therefore, some electrons may jump to conduction band and show some conductivity, electrical conductivity of semi-conductors increases with rise in “temperature”, since more electrons can jump to the conduction band. Substancesjike silicon and germanium show this type of behaviour and are called intrinsic semi-concuctors.
2. Extrinsic semi-conductors:
Their conductivity is due to the presence of impurities. They are formed by “doping”.
Doping : Conductivity of semi-conductors is too low to be of practical use. Their conductivity is increased by adding an appufiate amount of suitable impurity. This process is called “doping”. ‘
Doping can be done with an impurity which is electron rich of electron deficient. Extrinsic semi-conductors are of two types.
a) n-type semi-conductors:
It is obtained by adding trace amount of V group element (P, As, Sb) to pure Si or Ge by doping.
When P, As, Sb (or) Bi is added to Si or Ge some of the Si or Ge in the crystal are replaced by P or As atoms and four out of five electrons of P or As atom will be used for bonding with Si or Ge atoms while the fifth electron serve to conduct electricity.
b) p – type semi-conductors:
It is obtained by doping with impurity atoms containing less electrons i.e.. Ill group elements (B, Al, Ga or In).
When B or Al is added to pure Si or Ge some of the Si or Ge in the crystal are replaced by B or aZ atoms and four our of three electrons of B or AZ atom will be used for bonding with “Si” or Ge atoms while the fourth valence electron is missing is called electron hole (or) electron vacancy. This vacancy on an atom in the structure migrates from on atom to another. Hence it facilitates the electrical conductivity.
![]()
Question 13.
What are lyophilic and lyophobic sols ? Compare the two terms in terms of stability and reversibility.
Answer:
Lyophilic colloid:
The colloidal solution in which the dispersed phase has great affinity to the dispersion, medium is called a Lyophilic colloid or Lyophilic solution. Ex : Starch solution. The starch paste when dissolved in hot water, with stirring, the starch solution is formed. The starch particles (dispersed phase) has great affinity to water molecules (dispersion medium). So starch solution is a lyophilic solution or lyophilic colloid.
Lyophobic colloid :
The colloidal solution in which there exists not much affinity between the dispersed phase and dispersion medium, it is called a Lyophobic colloid or Lyophobic solution. Ex : Gold solution.
Gold rods are placed in water containing alkali. Electric arc is applied between gold rods. The gold particles dissolves in water, to give gold solution. Gold’particles (dispersed phase) have not much affinity towards water (dispersion medium). So this is a Lyophobic solution or Lyophobic colloid.
Question 14.
Out line the principles of metals by the following methods
(a) Zone refining
(b) Poling
Answer:
a) Zone refining :
- Zone refining is based on the principle that the impurities are more soluble in the melt than in the solid state of the metal.
- A circular mobile heater is fixed at one end of a rod of impure metal.
- The molten zone moves along with the heater moves forward the pure metal crystallises out of the melt and the impurities pass into the adjacent molten zone.
- The above process is repeated several times and the heater is moved in the same direction from one end to the other end. At one end impurities get concentrated. This end is cut off.
- This method is very useful for producing semiconductor grade metals’of very high purity. Eg : Ge, Si, B, Ga etc.
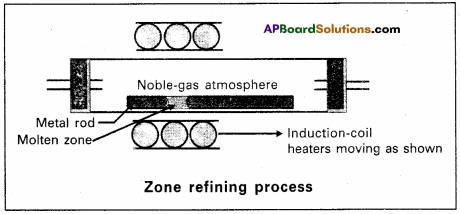
(b) Poling :
When the metals are having the metal oxides as impurities this method is employed. The impure metal is melted and is then covered by carbon powder. Then it is stirred with green wood poles. The reducing gases formed from the green wood and the carbon, reduce the oxides to metal. Eg : Cu & Sn metals are refined by this method.
Question 15.
Write IUPAC names of the following.
(a) [CU(NH3)4]SO4
(b) K3[Cr(C2O4)3]
(c) [co(scN)4-2
(d) [pt cl2 (NH3)2]
Answer:
(a) [CU(NH3)4]SO4 – Tetra amine copper (11) sulphate
(b) K3[Cr(C2O4)3] – Potassium trioxalato chromate (11)
(c) [CO(SCN)4]-2 – Tetra thiocyano cobalt (11) ion
(d) [Pt Cl2 (NH3)2] – Diamine dichloro platinum (11)
![]()
Question 16.
Write the names of the monomers in the polymers.
(a) Bakelite
(b) Polyvinyl chloride
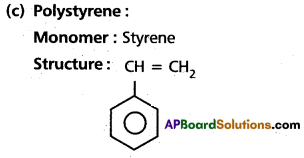
(c) polystyrene
(d) Teflon
Answer:
(a) Bakelite
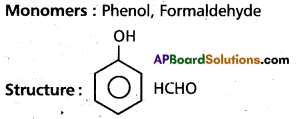
(b) Polyvinyl chloride
Monomers : Vinyl chloide
Structure : CH2 = CH – Cl
(d) Teflon:
Monomers : Tetrafluoro erthylene
Structure : CF2 = CF2
Question 17.
Define the following :
(a) Racemic mixture
(b) Enantioaers
Answer:
(a) Racemic mixture:
Equal portions of Enantiomers combined to form an optically inactive mixture. This mixture is called racemic mixture.
- Here rotation due to one isomer will be exactly cancelled by the rotation of due to other isomer.
- The process of conversion of enantiomer into a racemic mixture is called as racemisation.

(b) Enantiomers : The stereo isomers related to each other as non-superimposable mirror images are called enantiomers.
- These have identical physical properties like melting point, boiling point, refractive index etc.
- They differ in rotation of plane polarised light.
Question 18.
Explain the following.
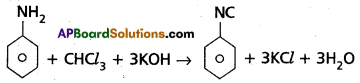
(a) Carbyl amine reaction
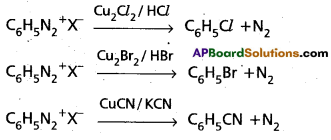
(b) Sand meyer reaction
Answer:
(a) Carbyl amine reaction : Aniline reacts with chloroform in presence of alc.kOH to form phenyl iso cyanide. This reaction is called carbylamine reaction.
(b) Sandmeyer reaction : Formation of chloro benzene, Bromo benzene (or) cyano benzene from benzene diazonium salts with reagents Cu2Cl2/HCl, Cu2Br2/HBr, CuCN/KCN is called sandmeyer’s reaction.
Question 19.
(a) How is nitric acid manufactured by Ostwald’s process ?
(b) How does chlorine reacts with the following and write equations,
(i) H2S
(ii) l2
(iii) Ca(OH)2
(iv) H20
Answer:
(a) Ostwald’s process ; Ammonia, mixed with air in 1:7 or 1:8, when passed over a hot platinum gauze catalyst, is oxidised to NO mostly (about 95%). The reaction is,
![]()
(b) i) H2S : Cl2 reacts with HsS and forms HsS and ‘S’. Cl2 + H2S → 2 HCl + S
ii) I2 : Cl2 reacts with Iodine to form ICl.
I2 + Cl2 → 2 ICl(Equi molar)
iii) Ca(OH)2 : Chlorine reacts with dry slaked lime and forms bleaching powder.
Ca(OH)2 + Cl2 → CaOCl2 + H20
iv) H2O : Chlorine reacts with water and forms chlorine water.
Cl2+ H2O → HCl + HOCl
HOCl is unstable and decompose to give nascent oxygen.
HOCl → HaCl + (O)
![]()
Question 20.
(a) Give detailed account of the collision theory of reaction
rates of bimolecular gaseous reactions.
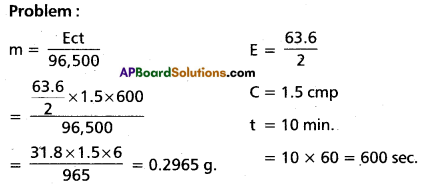
(b) State Faraday’s first law. A solution of CuSO4 is electrolysed for 10 min with a current of 1.5 amp. What is the mass of copper deposited at the cathode?
Answer:
(a) The reaction molecules are assumed to be hard spheres.
- The reaction is postulated to occur when molecules collide with each other.
- The number of collisions per second per unit volume of the reaction mixture is known as collision frequency (Z).
- For a bimolecular elementary reaction A + B → products
Rate = ZAB.e-E<a/RT ZAB = collision frequency. - All collisions do not lead to product formation.
- The collisions with sufficient kinetic energy (Threshold energy) are responsible for product formation. These are called as effective collisions.
- To account for effective collisions a factor p called to probability factor or steric factor is introduced.
Rate = P ZAB.e-Ea/RT - The proper orientation, of reactant molecular lead to bond formation where as improper orientation makes them back and no products are formed.
(b) The amount of chemical reaction which occurs at any electrode during electrolysis is proportional to the quantity of current passing through the electrolyte.
(Or)
The mass of the substance deposited at an electrode during the electrolysis of electrolyte is directly proportional to quantity of electricity passed through it.
m ∝ Q; m ∝ = c × t
m = ect: m = \(\frac{\text { Ect }}{96,500}\)
e = electrochemical equivalent
t = time in seconds
c = Current in amperes
E = Chemical equivalent
Question 21.
Explain the following,
(a) Acylation
(b) Esterification
(c) Gatterman – koch reaction
(d) Decarboxylation.
Answer:
(a) Acylation :
Aniline reacts with acetyl chloride/acetic acid/acetic anhydride to form acetanilide.
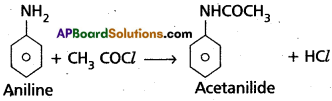
(b) Esterification:
Alcohol reacts with carboxylic acid in presence of acid to form ester. This reaction is called esterification.
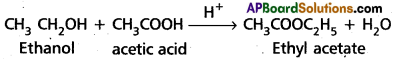
(c) Gatterman – koch reaction:
Benzene reacts with Co/HCl in presence of anhydrous AlCl3 to form benzaldehyde.
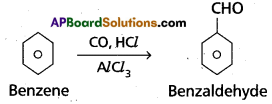
(d) Decarboxylation : Sodium salt of carboxylic acid undergo reaction with NaOH + CaO and removes CO2 from the compound. This reaction is called decarboxylation.
![]()