Access to a variety of TS Inter 2nd Year Chemistry Model Papers and TS Inter 2nd Year Chemistry Question Paper March 2020 helps students overcome exam anxiety by fostering familiarity.
TS Inter 2nd Year Chemistry Question Paper March 2020
Note : Read the following instructions carefully.
- Answer all questions of Section – ‘A’. Answer any six questions in Section – ‘B’ and any two questions in Section – ‘C’.
- In Section – A, questions from Sr. Nos. 1 to 10 are of “Very Short Answer Type”. Each question carries two marks. Every answer may be limited to 2 or 3 sentences. Answer all these questions at one place in the same order.
- In Section -”’8′, questions from Sr. Nos. 11 to 18 are of “Short Answer Type”. Each question carries four marks. Every answer may be limited to 75 words.
- In Section – ‘C’, questions from Sr. Nos. 19 to 21 are of “Long Answer Type”. Each question carries eight marks. Every answer may be limited to 300 words.
- Draw labelled diagrams wherever necessary for questions in Section – ‘B’ and ‘C’.
Section – A
Note : Answer all the questions.
Question 1.
State Raoult’s law.
Answer:
Raoult’s law for volatile solute : For a solution of voltile liquids, the partial vapour pressure of each omponent of the solution is directly proportional to its mole fraction present in solution. Raoult’s law for non-volatile solute : The relative lowering of vapour pressure of dilute solution containing non-volatile solute is equal to the mole fraction of solute.
Question 2.
State Faraday’s Law.
Answer:
Faraday’s First Law:
The amount of chemical reaction which occurs at any electrode during electrolysis is proportional to the quantity of current passing through the electrolyte.
OR
The mass of the substance deposited at an electrode during the electrolysis of electrolyte is directly proportional to quantity of electricity passed through it.
m α Q; m α c × t
m = ect;
m = \(\frac{\text { Ect }}{96,500}\)
e = electrochemical equivalent
t = time in seconds
c = Current in amperes
E = Chemical equivalent
Faraday’s Second Law: The amounts of different substances liberated when the same quantity of electricity is passed through the electrolytic solution are porportional to their chemical equivalent weights, m α E
Question 3.
Write ores with formulae of the following metals :
(i) Aluminium
(ii) Iron
Answer:
i) Ores of Aluminium Bauxite – A12O3.2H2O
Cryolite – Na3Al F6
ii) Ores of Iron Haematite – Fe2O3
Magnetite – Fe3O4
Question 4.
What is Lanthanoid contraction?
Answer:
Lanthanide contraction : The slow decrease of Atomic in lanthanides with increase in atomic number is called Lanthanide contraction.
![]()
Question 5.
What are Polymers? Give example.
Answer:
Polymer : A large molecular weight complex compound which is formed by the repeated combination of simple units (monomers) is called polymer. E.g. : Nylon 6, 6, Buna – S, rubber etc …..
Question 6.
What is vulcanization of rubber?
Answer:
Vulcanization of rubber : The process of heating the raw rubber with sulphur (or) with sulphur compounds to improve its physical properties is called vulcanization of rubber.
Question 7.
What are antacids ? Give example.
Answer:
Antacids : Chemicals that remove the excess of acid in the stomach and maintain the pH to normal level are antacids. E.g. : (Dmeprozole, Lansoprozole etc.,
Question 8.
What is tincture of iodine? What is its use?
Answer:
Tincture of iodine (antiseptic) is a mixture of 2-3% Iodine solution in alcohol – water.
![]()
Question 9.
What are Enantiomers?
Answer:
Enantiomers : The stereo isomers related to each other as non-superimposable mirror images are called enantiomers.
- These have identical physical properties like melting point, boiling points refractive index etc.
- They differ in rotation of plane polarised right.
Question 10.
What are ambident nucleophiles?
Answer:
Ambident nucleophiles : The nucleophiles which are able to attack at two (or) more different sites are called ambident nucleophiles.
Eg: Alkyl halides react with AgCN to form alkyl cyanide and isocyanides.
Img-1
Section – B
Question 11.
Derive Bragg’s equation.
Answer:
Derivation of Bragg’s equation : When X-rays are incident on the crystal or plane, they are diffracted from the lattice points (lattice points may be atoms or ions or molecules).
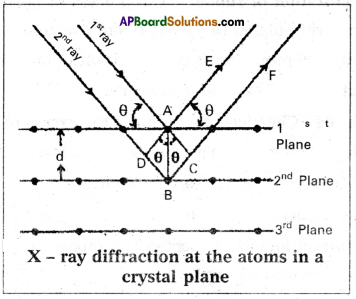
In the crystal the lattice points are arranged in regular pattern. When the waves are diffracted from these points, the waves may be constructive or destructive interference.
The 1st and 21nd waves reach the crystal surface. They undergo constructive interference. Then from the figure 1st and 2nd rays are parallel waves. So, they travel the same distance till the wave form AD. The second ray travels more than the first by an extra distance (DB + BC) after crossing the grating for it to’interfere with the first ray in a constructive manner. Then only they can be in the same phase with one another. If the two waves are to be in phase, the path difference between the two ways must be equal to the Wavelength (λ) or integral multiple of it (n λ, where n = 1, 2, 3,……)
(i.e.,) nλ = (DB + BC)
[where n = order of diffraction]
DB = BC = d sin θ
θ = angle of incident beam,
(DB + BC) = 2d sin θ
d = distance between the planes
nλ = 2d sin θ This relation is known as Bragg’s equation.
Question 12.
Define molarity. Calculate the molarity of a solution con-taining 5 g of NaOH pi 450 ml solution.
Answer:
Molarity : The number of moles of solute dissolved in one litre of solution is called molarity

Question 13.
What are different types of adsorption ? Give any four differences between characteristics of these different types.
Answer:
Adsorption process is divided into two types :
1) Physiosorption
2) Chemisorption
Distinguishing characteristics of Physiosorption Chemisorption are given in the following table:
| Physisorption | Chemisorption |
| 1. This process is weak, due to vander Waall’s forces. | 1. This process is strong, due to chemical forces. |
| 2. The process is reversible | 2. The process is irreversible |
| 3. This is a quick process i.e. takes place quickly | 3. This is a slow process. |
| 4. The process decreases with increase of tempe¬rature | 4. The process increases with increase of temperature. |
| 5. This is a multilayered process | 5. This is a unilayered process |
| 6. The process depends mainly on the nature of the absorbent. | 6. The process depends both on the nature of adsorbent and adsorbate. |
![]()
Question 14.
Explain the purification of sulphide ore by Froth floatation
method.
Answer:
This method is used to concentrate sulphide ores.
- In this process a suspension of the powdered ore is made with water.
- A roating paddle is used to agitate the suspension and air is blown into the suspension in presence of an oil.
- Froth is formed as a result of blown of air, which carries the mineral particles.
- To the above slurry froth collectors and stabilizers are added.
- Collectors like pine oil enhance non-wettability of the mineral particles.
- Froth stabilizers like cresol stabilize the froth.
- The mineral particles wet by oil and gangue particles wet by water.
- The froth is light and is skimmed off. The ore particles are then obtained from the forth.
By using depressants in froth floatation process, it is possible to separate a mixture of two sulphide ores.
Eg: In the ore containing ZnS and PbS, the depressant used in NaCN. It prevents ZnS from coming to the froth, but allows PbS to come with the froth.
Question 15.
Explain the terms :
(a) Ligand
(b) Co-ordination number
(c) Co-ordination entity
(d) Central metal atom/ion
Answer:
i) Ligand:
The ions or molecules bound to the central atom/ ion in the co-ordination entity are called ligands. These may be:
a) simple ions such as Cl–
b) small molecules such as H2O or NH3
c) large molecules such as H2NCH2CH2NH2 or N(CH2CH2NH2)3 or even
(d) macro – molecules, such as proteins. On the basis of the number of donor atoms available for co-ordination, the ligands can be classified as :
a) Unidentate : One donor atom, Eg : NH3, :CI: etc.
b) Bidentate : Two donor atoms Eg : H2NCH2CH2NH2 (ethane-1, 2-diamine or en), C2O42- (oxalate), etc.
c) Polydentate : More than two donor atoms, Eg:N(CH2CH2NH2)3 EDTA, etc.
ii) Co-ordination number:
The co-ordination number (CN) of metal ion in a complex can be defined as the number of ligands or donor atoms to which the metal is directly bonded.
Eg : In [PtCl6]2-, CN of Pt = 6,
In [Ni(NH3)4]2+, CN of Ni = 4..
iii) Co-ordination entity:
A central metal atoms or ion bonded to a fixed number of molecules or ions (ligands) is known as co-ordination entity. For example [CoCl3(NH3)3]. Ni(CO)4], etc.
iv) Central metal atom/ion : In a co-ordination entity, the atom/ion to which a fixed no. of ions/groups are bound in a definite geometrical arrangement around it is called central metal atom or ion.
Eg: K4[Fe(CN)6]’Fe’ is central metal.
Question 16.
How are XeF2 and XeF4 prepared? Give their structure.
Answer:
Xenon forms the binary fluorides XeF2, XeF4, XeF6 as follows. These are formed by direct combination of Xe and F2.
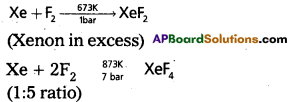
a) Structure of XeF2 :
1) In XeF2 central atom is Xe’.
2) ‘Xe’ undergoes sp3d hybridisation in it’s 1 st excited state
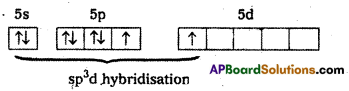
3) Shape of molecule is linear
4) Xe form two a – bonds with two fluorines (sp3d2-2pz(F) overlap)
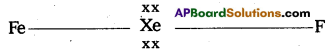
b) Structure of XeF4
1. Central atom in XeF4 is ‘Xe’
2) ‘Xe’ undergoes sp3d2 hybridisation in it’s 2nd excited state
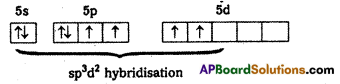
3) Shape of the molecule is square planar with bond angle 90° and bond length 1. 95A.
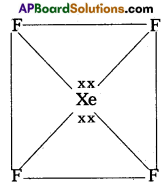
Question 17.
What are hormones ? Give one example for each.
(a) Steroid hormone
(b) Polypeptide hormones and
(c) Amino acid derivatives.
Answer: Hormones : Hormone is defined as an “organic compound synthesised by the ductless glands of the body and carried by the blood stream to another part of the body for its funtion”. Ex: testosterone, estrogen
- Example for steroid hormones : Testosterone, Estrogen
- Example for poly peptide hormones : Insulin
- Example for Amino acid activities: Thyroidal hormones thyroxine.
Question 18.
Write the IUPAC names of the following compounds :
(a) NH2 – CH2 – CH = CH2
(b) C2H5 – N – CH2 – CH2 – CH2 – CH3

Answer:
a) NH2 – CH2 – CH = CH2 = Prop 2 – ene 1 – Amine.
b) C2H5 – N – CH2 – CH2 – CH2 – CH3 = M, N – Di Ethyl Buton amine.
Section – C
Question 19.
a) What are Galvanic Cells ? Explain the working of a gal-vanic cell with a neat sketch taking Daniel cell as ex-ample.
Answer:
Galvanic cell: A device which converts chemical energy into electrical energy by the use of spontaneous redox reaction is called Galvanic cell (or) voltaic cell.
Eg: Daniell cell.
Daniell cell: It is a special type of galvanic cell. It contains two half cells in the same vessel.
The vessel is divided into two chambers. Left chamber is filled with ZnSO4 (aq) solution and Zn – rod is dipped into it. Right chamber is filled with aq. CuSO4 solution and a copper rod is dipped into it. Process diaphragm acts as Salt bridge. The two half cell’s are connected to external batteiy.
Cell reactions:
Ion Zn/ZnSO4 half cell, oxidation, reaction occurs
Zn → Zn2+ + 2e
Ion Cu/CuS4 half cell, reduction reaction occurs.
Cu+2 + 2e– → Cu
The net cell reaction is
Zn + Cu+2 ⇌ Zn+2 + Cu
Cell is represented as Zn/Zn+2//Cu2+/Cu
b) What are fuel cells ? Give the construction of H2, O2 fuel cell.
Answer:
A fuel cell is a galvanic cell in which the chemical energy of fuel – oxidant system is converted directly into electrical energy.
- Conventional Galvanic cell converts chemical energy into electrical energy by spontaneous redox reactions.
- Fhel cell convert energy of combustion of fuels like hydrogen, methane etc., into electrical energy. These cause less pollution.
H2-O2 fuel cell: ln this cell, hydrogen and oxygen are bubbled through porous carbon electrodes into Cone. NaOH solution. Electrodes are embedded with suitable catalysts. The electrode reactions are :
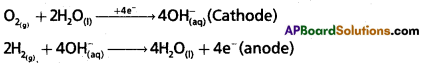
Overall reaction : 2H2(g) + O2(g) → 2HO2O(l)
The cell functions as long as the reacting gases are in supply. The heat of‘combustion is directly converted into electrical energy.
![]()
Question 20.
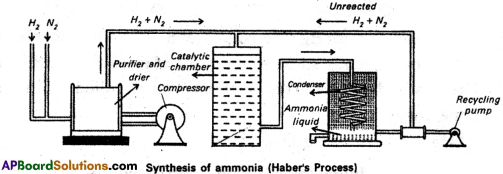
a) How is Ammonia manufactured by Haber’s process?
Answer:
In Haber process ammonia is directly synthesised from elements (nitrogen and hydrogen). The principle involved in this is
N2(g) + 3H2(g) ⇌ 2NH3 + 92.4 kJ. This is a reversible exothermic reaction.
According to Le Chatelier’s principle favourable conditions for the better yield of ammonia are low temperature and high pressure. But the optimum conditions are:
Temperature : 720k
Pressure : 200 atmospheres
Catalyst : Finely divided iron in the presence of molybdenum (Promoter).
Procedure: A mixture of nitrogen and hydrogen in the volume ratio 1 : 3 is heated to 720 – 775 K at a pressure of 200 atmospheres is passed over hot finely divided iron mixed with small amount of molybdenum as promotor. The gases coming out of the catalyst chamber consists of 10-20% ammonia gas are cooled and compressed, so that ammonia gas is liquified, and the uncondensed gases are sent for recirculation,
a) Aq.ZnS4 reacts with ammonia aqueous solution to form white ppt Of zinc hydroxide.
NH3(ab) + H2O(l) ⇌ NH4(ab)+ + OH(ab)–
ZnSO4(ab)– + 2NH3(ab) → Zn(OH)2(s) + (NH4)2SO(ab)
b) Aq.CuSO4 reacts with ammonia to form a deep blue complex.
![]()
c) Solid AgCl reacts with ammonia to form a colourless complex.
![]()
b) How does Ozone reacts with the following:
(i) pbS
(ii) KI
(iii) Hg
(iv) Ag
Answer:
Preparation of Ozone :
A slow dry stream of oxygen under silent electric discharge to form ozone (about 10%). The product obtained is known as ozonised oxygen.
3O2 → 2O3 ∆H° = 142 kJ/mole
- The formation of ozone is an endothermic reaction.
- It is necessary to use silent electric discharge in the preparation of Oa to prevent its decomposition.
i) Reaction with PbS : Black lead sulphide oxidised to white lead sulphate in presence of ozone.
PbS + 4O3 → PbSO4 + 4O2
ii) Reaction with KI: moist KI is oxidised to Iodine in presence of ozone.
2KI + H2O + O3 → 2KOH + I2 + O2
iii) Reaction with Hg: Mercury loses it’s lustreness, meniscus and consequently sticks to the walls of glass vessel when it reacts with ozone. This phenomenon is called tailing of mercury.
2Hg + O3 → Hg2O + O2
Question 21.
Explain the following reactions with equations.
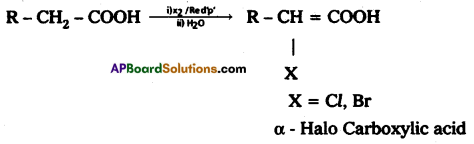
a) Hell-Volhard-Zelinsky reaction (HVZ):
Answer:
Carboxylic acids having α – hydrogens are halogenated at the α – position on treatment with chlorine or bromine in presence of small amount of red phosphorous to give α – halo carboxylic acids.
This reaction is named as Hell – volhard – Zelinsky (HvZ) reactions
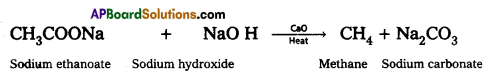
b) Decarboxylation:
Answer:
Carboxylic acids lose carbon dioxide molecule to produce hydrocarbons on heating their sodium salts with sodalime (a mixture of NaOH & CaO in ratio 3:1). This reaction is called decarboxylation.
c) Aldol condensation
Answer:
When aldol condensation is carried out between two different aldehydes and (or) ketones, it is called cross aldol condensation.
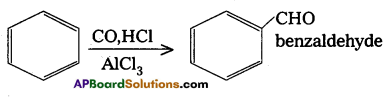
d) Gatterman-Koch reaction
Answer:
Gater man – Koch reactioni:
Benzene or its derivatives treated with CO and HCl in presence of A1Cl3 gives Benzaldehyde.