Timed practice with TS 10th Class Physical Science Model Papers Set 4 is crucial for improving speed and efficiency during exams.
TS 10th Class Physical Science Model Paper Set 4 with Solutions
Time: 1 Hour 30 minutes
Maximum Marks: 40
General Instructions:
- Read the question paper and understand every question thoroughly and write answers in given 1.30 hrs. time.
- 3 very short answer questions are there in section – I. Each question carries 2 marks. Answer all the questions. Write answer to each question in 3 to 4 sentences.
- 3 short answer questions are there in section – II. Each question carries 3 marks. Answer all the questions. Write answer to each question in 5 to 6 sentences.
- 3 essay type answer questions are there in section – III. Each question carries 5 marks. Answer all the questions. Write answer to each question in 8 to 10 sentences. Internal choice is given in this section.
Part – A (30 Marks)
Section – I (3 × 2 = 6 Marks)
Instructions :
- 3 Very short answer questions are there in section – I.
- Answer ALL the questions. Each question carries 2 marks.
- Write answer to each question in 3 to 4 sentences.
Question 1.
Suggest a new use with a spherical mirror.
Answer:
- Concave mirrors ate used in long focal length camera lenses.
- In ‘Super snooper’ to focus and magnify sound from a great distance onto a microphone pickup.
- At one end of some gas LASERS to focus the emerging beam of light.
- In the telescopes to focus the very weak radio waves onto the suspended antenna.
Question 2.
How can we appreciate the working of “Iris” ?
Answer:
- Iris consists of muscles which helps in controlling the amount of light entering the eye through pupil.
- Increase of low light the iris makes the pupil to expand and allow more light to enter the eye.
- In case of bright (or) excess light the iris makes the pupil to contract in order to decrease the amount of light entering the eye. The iris consists of muscles that expand and contract the pupil.
![]()
Question 3.
Give some examples for corrosion.
Answer:
Examples for corrosion:
- The rusting of iron (Iron oxide)
- Tarnishing of silver (Silver sulphide)
- Development of green coating on copper (Copper carbonate) and bronze.
Section – II (3 × 3 = 9 Marks)
Instructions :
- 3 Short answer questions are there in section – II.
- Answer ALL the questions. Each question carries 3 marks.
- Write answer to each question in 5 to 6 sentences.
Question 4.
Some elements belonging to second period of periodic table, and their atomic radii are given below. Observe them and write answers.

a) Write the elements in the ascending order of their atomic radii.
b) Which of the 2nd period elements closer to the configuration of inert gas ?
c) Which is the outermost orbit of all these elements ?
d) Which elements atomic size is bigger. Beryllium or Carbon ? Why ?
Answer:
a) The ascending order of atomic sizes is O, N, C, B, Be and Li.
b) Lithium has closest inert gas configuration i.e., 1s2 2sl. Its nearest inert gas is helium.
c) The outermost orbit for all these elements is second orbit.
d) Beryllium has more atomic size than Carbon. Because when we move across a period the atomic number increases. So nuclear attraction of outermost orbit increases. So carbon has lesser size than Beryllium.
Question 5.
Elements X, Y have atomic numbers 9 and 12 respectively. Which one of these
a) forms negative ion ?
b) forms positive ion ?
Answer:
a)
i) An element whose atomic number 9 is fluorine.
Electronic configuration is 1s22s22p5.
ii) To attain octet configuration one electron is required. So, it possesses high electronegativity. By gaining one electron it becomes FΘ(negative ion).
F + leΘ → FΘ
iii) Then the electronic configuration of F is 1s22s2p6.
b) iv) An element whose atomic number 12 is magnesium. Electronic configuration is 1s22s22p63s2.
v) To attain octet configuration two electrons it has to loose. So, it possesses high electropositivity. By loosing two electrons it becomes Mg2+(Positive ion).
Mg – 2e- → Mg2+
vi) Then the electronic configuration of Mg2+ is 1s22s22p6.
Question 6.
Draw the structures of the following, a) Ethanoic acid b) Propanol c) Propene
Answer:
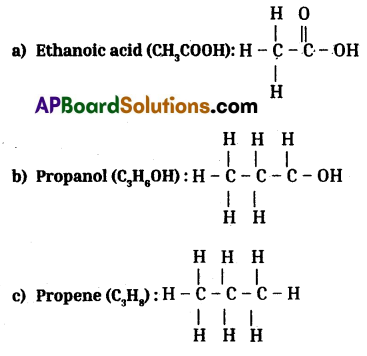
![]()
Section – III (3 × 5 = 15 Marks)
Instructions :
- 3 Essay answer questions are there in section.
- Answer ALL the questions. Each question carries 5 marks.
- Internal choice is given in this section.
- Write answer to each question in 8 to 10 sentences.
Question 7.
What is the difference between dis-placement and double displacement reactions ? Write equations for these reactions.
(OR)
Describe how sodium hydroxide is obtained from common salt.
Answer:
- Displacement reaction: The reaction in which an element displaces another element from a substance is called “displacement reaction.” It is represented as
AB + C → AC + B
Ex: Zn + 2HCl → ZnCl2 + H2 - In displacement reaction one element displaces another element from its compound and takes its place therein.
- Here in above equation hydrogen has displaced from acids by metals.
- Double displacement: The reaction in which the constituent atoms of the reaction interchange by the decomposition of the two reactants, to produce new compounds are called double displacement reactions.
They are generally represented as follows.
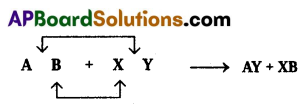
Ex:
1) MgCl2 + 2KOH → Mg(OH)2 + 2KCl
2) HCl + NaOH → NaCl + H2O
3) Pb(NO3)2 + 2KI → PbI2 + 2KNO3
(OR)
- When electricity is passed through an aqueous solution of sodium chloride (called brine), it decomposes to form sodium hydroxide.
- The process is called chloralkali process because of the products formed chlor for chlorine and alkali for sodium hydroxide
2NaCl(aq) + 2H2O(l)
2NaOH(aq) + Cl2(g) + H2(g) - Chlorine gas is liberated at the anode and hydrogen gas at the cathode.
- Sodium hydroxide solution is formed near the cathode.
- The three products produced in this process are all useful.
Question 8.
Draw ray diagrams of image formed by a convex lens at various distances.
(OR)
Explain Bohr – Sommerfeld model of an atom. What is the merit of this model ? What are its limitations?
Answer:
1) Object at infinity.
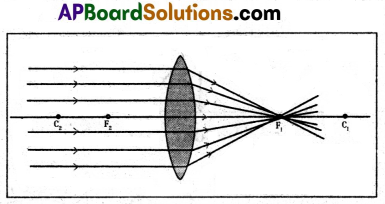
2) Object placed beyond the centre of curvature (2F).
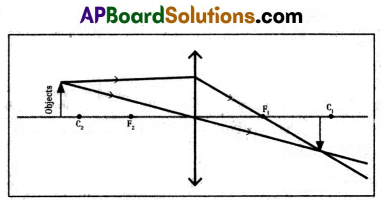
3) Object placed at the centre of curvature.
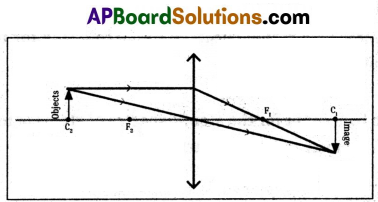
4) Object placed between C and F.
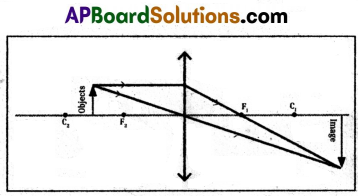
5) Object at the focal point.
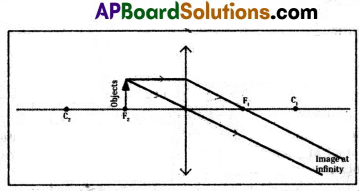
6) Object placed between F and P.
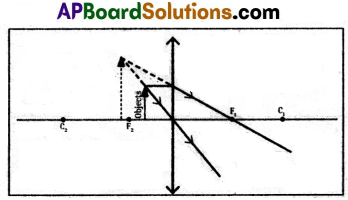
(OR)
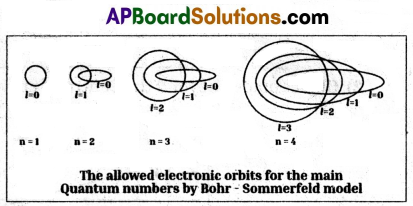
- In an attempt to account for the structure of line spectra, Sommerfeld modified Bohr’s atomic model by adding elliptical orbits.
- While retaining the first of Bohr’s circular orbit as such, he added one elliptical orbit to Bohr’s second orbit, two elliptical orbits to Bohr’s third orbit etc.
- Nucleus of the atom is one of the principal foci of these elliptical orbits because periodic motion under the influence of a central force will lead to elliptical orbits with the force situated at one of the foci.
Merit : Bohr – Sommerfeld model successful in accounting for the fine line structure of hydrogen atomic spectra.
Limitations:
- This model failed to account for the atomic spectra of atoms of more than one electron.
- It did not explain Zeeman and Stark effects.
![]()
Question 9.
Derive an expression to measure emf of a battery.
(OR)
Give one application of Faraday’s laws of induction in daily life.
Answer:
Electromotive force (emf) is defined as the work done by the chemical force’ to move unit pos¬itive charge from negative terminal to positive terminal of the battery.
-
- Let this chemical force be Fc.
- This chemical force does some work to move a negative charge ‘q’ from positive terminal to negative terminal against the electric force Fe Let this work be ‘W’.
- The work done by the chemical force to move 1 coulomb of charge from the terminal to negative terminal is given by \(\frac{w}{q}=\frac{F_c d}{q}\).
Where ‘d’ is the distance between the terminals.
If we assume Fc = Fe, then \(\frac{w}{q}=\frac{F_c d}{q}\)
This \(\frac{w}{q}\) is known as emf (e) of the battery.
∴ emf(s) = \(\frac{w}{q}=\frac{F_c d}{q}\)
This S.I. unit of emf is ‘volt’ and is measured using voltmeter.
(OR)
Now-a-days we can’t imagine the modern world with out electricity.
The generation of electricity utilizes the principle of electromagnetic induction. Transfer of generated power is possible with transformer the most important electrical device in our day to day life.
In bed lamps we are using step down transformer and to increase the voltage we are using step-up transformer.
Transformer used the principle of mutual induction i.e., when current flowing through a coil or magnetic flux linked with a coil changes an induced emf is produced in other coil.
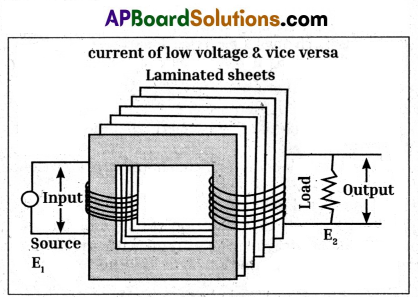
Part – B (10 × 1 = 10 Marks)
Instructions:
- Answer ALL the questions.
- Each question carries 1 mark. .
- In this section there are 4 options (A / B / C / D) to each question. Choose the appropriate answer and write the answer in the brackets given against the question. Part – B must be attached to the answer booklet of Part-A.
Question 1.
The image formed by a convex mirror is always
A) real and magnified
B) real and diminished
C) virtual and diminished
D) virtual and magnified
Answer:
C) virtual and diminished
Question 2.
CaO reacts with water to form ………
A) CaCO3
B) Ca(OH)2
C) CaCl2
D) Ca(NO3)2
Answer:
B) Ca(OH)2
Question 3.
Identify the pair of pH values of strong acid and strong base in the following.
A) (6, 14)
B) (1, 8)
C) (7, 7)
D) (2, 14)
Answer:
D) (2, 14)
Question 4.
We are using tooth paste to clean our mouth and to avoid tooth decay. The nature the tooth paste is …………..
A) Acidic
B) Base
C) Neutral
D) Amphoteric
Answer:
C) Neutral
Question 5.
The acid forms in stomach is
A) HCl
B) H2SO4
C) CH3COOH
D) HNO3
Answer:
A) HCl
Question 6.
Which of the following lens act as converging lens ?
A) Biconvex lens
B) Plano convex lens
C) Concave convex
D) All the above
Answer:
D) All the above
![]()
Question 7.
What happens to the focal length of a convex lens when it is kept in water ?
A) decreased
B) we can’t tell
C) constant
D) increased
Answer:
D) increased
Question 8.
The sky looks blue on a clear, sunny day because of ……………….
A) dispersion of light
B) scattering of light
C) reflection of light
D) refraction of light
Answer:
B) scattering of light
Question 9.
Ionization energy is expressed in
A) KJ mol-1
B) J/K mol-1
C) JK mol-1
D) K/J mol-1
Answer:
A) KJ mol-1
Question 10.
Rusting of iron takes place in
A) ordinary water
B) both ordinary and distilled water
C) distilled water
D) none of the above
Answer:
B) both ordinary and distilled water