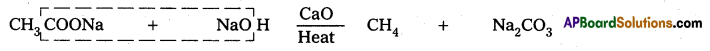Access to a variety of TS Inter 2nd Year Chemistry Model Papers and TS Inter 2nd Year Chemistry Question Paper March 2017 helps students overcome exam anxiety by fostering familiarity.
TS Inter 2nd Year Chemistry Question Paper March 2017
Note : Read the following instructions carefully.
- Answer all questions of Section – ‘A’. Answer any six questions in Section – ‘B’ and any two questions in Section – ‘C’.
- In Section – A, questions from Sr. Nos. 1 to 10 are of “Very Short Answer Type”. Each question carries two marks. Every answer may be limited to 2 or 3 sentences. Answer all these questions at one place in the same order.
- In Section -”’8′, questions from Sr. Nos. 11 to 18 are of “Short Answer Type”. Each question carries four marks. Every answer may be limited to 75 words.
- In Section – ‘C’, questions from Sr. Nos. 19 to 21 are of “Long Answer Type”. Each question carries eight marks. Every answer may be limited to 300 words.
- Draw labelled diagrams wherever necessary for questions in Section – ‘B’ and ‘C’.
Section – A
Note : Answer all the questions.
Question 1.
What is metallic corrosion ? Give ail example.
Answer:
Metallic corrosion: The natural tendency of conversion of a metal to its mineral compound form on interaction with environment is known as metallic corrosion. Eg: 1) Rusting of iron
Chemical equation : 2Fe(S) + O2(g) + 4H+(aq) → 2Fe+2(aq) + 2H2O(l).
Question 2.
What is reverse osmosis ? What is practical utility?
Answer:
Reverse osmosis : The direction of osmosis can be reversed if a pressure larger than the Osmotic pressure is applied to the solution side i.e., pure solvent flows out of the solution through the semi permeable membrane. This is called reverse osmosis.
Practical utility:
Reverse osmosis is used in the purification of water.
Reverse osmosis is used in the food processing industry.
![]()
Question 3.
Give the composition of the following alloys :
a) Brass
b) German silver
Answer:
a) Composition of Brass : 60 – 80% Cu, 20 – 40% Zn
b) Composition of German silver : 50 – 60% Cu 10 – 30% Ni, 20 – 30% Zn partial hydrolysis of xeF6 gives xeF4
Question 4.
How is XeOF4 prepared? Describe its molecular shape.
Answer:
xeF6 undergo partial hydrolysis to form xeoF4

xeF6 + H2O → xeoF4 + 2HF
Structure of xeF4 :
xe is the central atom is xeoF4 and xe undergoes SP3d2 hybridisation. Shape of the molecule is square pyramid.
Question 5.
Give two reactions in which transition metals or their compounds acts as catalysts.
Answer:
In Haber’s process for the preparation of Ammonia catalyst is Iron.
![]()
In contact process for the preparation of SO3 catalyst is pt (or)V2O5
![]()
Question 6.
What are ambident nucleophiles?
Answer:
Ambident Nucleophiles:
The groups which possess two (or) more nucleophillic centres are called ambident nucleophiles.
Eg : Cyaride, Nitrites
Question 7.
What is stereochemical result of Sn1 and Sn2 reactions?
Answer:
The stereo chemical result of SN1 reaction is racemisation product and SN2 reaction is inversion product.
Question 8.
Give the structures of A and B.
![]()
Answer:
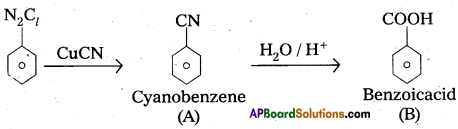
Question 9.
Explain Gatterman Reaction.
Answer:
Gatterman Reaction : Benzene diazonium chloride treated with corresponding halogen acid in presence of copper powder to form halobenzene.
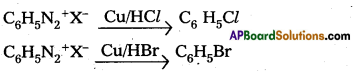
Question 10.
Write the reactions of F2 with water.
Answer:
Reaction of F2 with water : Flurine when passed through water produces O2 and O3
2F2 + 2H2O → 4HF + O2
3F2 + 3H2O → 6HF + O3
Section – B
Note : Answer any six questions.
Question 11.
Define molarity. Calculate the molarity of a solution containing 5g of NaOH in 500 ml of solution.
Answer:
Molarity: The number of moles of solute present in one litre of solution is called molarity (M).
Molarity(M) = \(\frac{\mathrm{W}}{\mathrm{GMW}} \times \frac{1000}{\mathrm{~V}(\mathrm{~m} l)}\)
units : Moles/lit.
Problem :
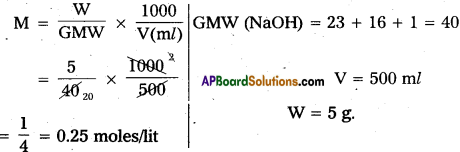
Question 12.
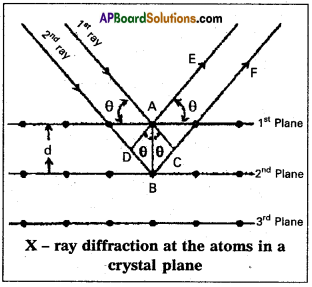
Derive Bragg’s equation.
Answer:
Derivation of Bragg’s equation: When X-rays are incident on the crystal or plane, they are diffracted from the lattice points (lattice points may be atoms or ions or molecules). In the crystal the lattice points are arranged in regular pattern. When the waves are diffracted from these points, the waves may be constructive or destructive interference.
The 1st and 2nd waves reach the crystal surface. They undergo constructive interference. Then from the figure 1st and 2nd rays are parallel waves. So, they travel the same distance till the wave form AD. The second nay travels more than the first by an extra distance (DB + BQ after crossing the grating for it to interfere with the first ray in a constructive manner. Then only they can be in the same phase with one another. If the two waves are to be in phase, the path difference between the two ways must be equal to the wavelength (λ) or integral multiple of it (n λ, where n = 1,2,3,…..)
(i.e.,) nλ = (DB + BC) [where n = order of diffraction]
DB = BC = d sin θ
θ = angle of incident beam,
(DB + BQ = 2d sin θ
d = distance between the planes
nλ = 2d sin θ This relation is known as Bragg’s equation.
![]()
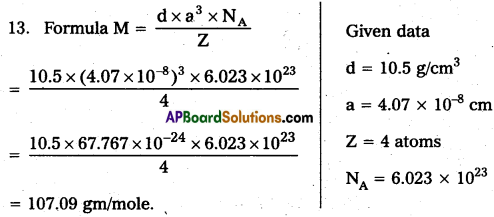
Question 13.
Give any four differences between physisorption and chemisorption.
Answer:
Question 14.
Explain briefly the extraction of aluminium from bauxite.
Answer:
The principal ore of aluminium, bauxite, usually contains SiO2, iron oxides and titanium oxide (TiO2) as impurities, concentration is carried out by digesting the powdered ore with a concentrated solution of NaOH at 473 – 523 K and 35 – 36 bar pressure. The way. Al2O3 is leached out as sodium aluminate (and Si02 too as sodium silicate) leaving the other impurities behind :
Al2O3 (S) + 2NaOH(aq) + 3H2O(l) → 2Na[Al(OH)4](aq)
The aluminate is alkaline in nature and is neutralised by passing CO2 gas and hydrated Al2O3 is precipitated. At this stage, the solution is seeded with freshly prepared samples of hydrated Al2O3 which induces the precipitation of Al2O3 xH2O
2 Na[Al(OH)4](aq) + CO2(g) → Al2O3 xH2O(s) +
2NaHCO3(aq)
The sodium silicate remains in the solution and the insoluble hydrated alumina is filtered, dried and heated to give pure Al2O3:
Question 15.
Write the formulas fQr the following coordination compounds.
a) Tetraammineaquachloro cobalt (III) chloride
b) Potassium tetrahydroxozincate (II)
c) Potassium trioxalatoaluminate (III)
d) Tetracarbonylnickel (0)
Answer:
a) Tetra amine aqua chloro cobalt (111) chloride.
[CO(NH3)4 (H2O)CZ] Cl2
b) Potassium tetra hydroxo zincate (11)
K2[Zn(OH)4]
c) Potassium trioxalato aluminate (111)
K3 [Al(C2O4)3]
d) Tetra carbonyl Nickel (O)
[Ni(CO)4]
![]()
Question 16.
Explain the purpose of vulcanization of rubber.
Answer:
- Natural rubber becomes soft at high temperatures and brittle at low temperatures. It shows high water absorption capacity.
- Natural rubber is soluble in non-polar solvents and is non-resistant to oxidising agents.
- To improve these physical properties rubber can be vulcanised.
- The process of heating the raw rubber with sulphur (or) with sulphur compounds to improve it’s physical properties is called vulcanisation of rubber.
- The vulcanized rubber has improved properties like elasticity, minimum water absorbing tendency, high resistance to chemical oxidation as well as organic solvents.
Question 17.
Give the sources of the following vitamins and name the dis¬eases caused by their deficiency.
a) A
b) D
c) E
d) K
Answer:
| Vitamin | Sources | Deficiency diseases |
| A | Fish oils, liver kidney | Night blindness,Redness in eyes. |
| D | Fish, oils, butter, milk, egg | Rickets in children, osteomalacia in adults |
| E | Wheat germ oil, egg yolk, green vegetables | Sterility |
| K | Green vegetables, Intestinal flora. | Blood coagulation is prevented |
Question 18.
Write notes on antiseptics and disinfectants.
Answer:
Antiseptics:
The chemical compounds that kill (or) prevent the growth of micro organism are called antiseptics. Antiseptics are the chemical substances applied on the living tissues such as wounds, cuts, ulcers and diseased skin surfaces. E.g. : Dettol, Bithional etc.
Phenol is used as antiseptic as well as disinfectant. 0.2% phenol is antiseptic. Dettol (antiseptic) is a mixture of chloroxylenol and terpineol. Tincture of iodine (antiseptic) is a mixture of 2 – 3% Iodine solution in alcohol-water.
Disinfectants :The chemical compounds used for killing (or) preventing the growth of microorganism are called . disinfectants.
- These, are applied to inanimate objects like floors, drainage systems etc…
E.g. : 4% aqueous solution of formaldehyde called formalin is a disinfectant. - 0.3 ppm chlorine aqueous solution is disinfectant.
- SO2 in very low concentration is disinfectant.
Phenol is used as antiseptic as well as disinfectant. 1 % phenol is disinfectant.
Section – C
Note : Answer any two questions.
Question 19.
a) How is Ammonia manufactured by Haber’s Process with a neat diagram?
b) Explain the reactions of the following with ozone,
a) C2H4
b)Kl
c) Hg
d) PbS
Answer:
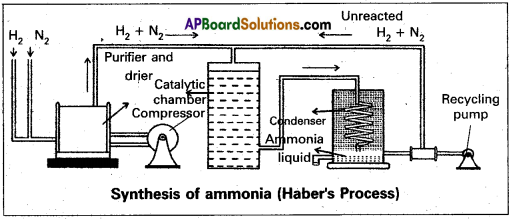
(a) In Haber process ammonia is directly synthesised from elements (nitrogen and hydrogen). The principle involved in this is N2(g) + 3H2 (g) ⇌ 2NH3 + 92.4 kj This is a reversible exothermic reaction.
According to Le Chatelier’s principle favourable conditions for the better yield of ammonia are low temperature and high pressure. But the optimum conditions are :
Temperature : 720k
Pressure : 200 atmospheres
Catalyst : Finely divided iron in the presence of molybdenum (Promoter).
Procedure :
A mixture of nitrogen and hydrogen in the volume ratio 1 : 3 is heated to 725 – 775K at a pressure of 200 atmospheres is passed over hot finely divided iron mixed with small amount of molybdenum as promotor. The gases coming out of the catalyst chamber consists of 10 – 20% ammonia gas are cooled and compressed, so that ammonia gas is liquified, and the uncondensed gases are sent for recirculation.
a) Aq. ZnSO4 reacts with ammonia aqueous solution to form white ppt of Zinc hydroxide.
NH3(aq) + H2(l) ⇌ NH4(aq)+ + OH–(aq)
ZnSO4(aq)+(aq) + 2NH4OH(aq) → Zn(OH)2(s) + (NH4)2 SO(aq) white ppt
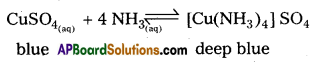
b) Aq.CuSO4 reacts with ammonia to form a deep blue complex.
c) Solid AgCl reacts with ammonia to form a colourless complex.
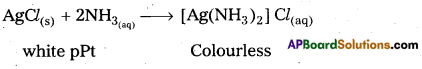
(b) Preparation of Ozone :
A slow dry stream of oxygen under silent electric discharge to form ozone (about 10%). The product obtained is known as ozonised oxygen.
3O2 → 2O3 ∆H° = 142kJ/mole
- The formation of ozone is an endothermic reaction.
- It is necessary to use silent electric discharge in the preparation of O3 to prevent its de composition
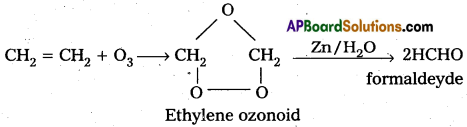
a) Reaction with C2H4 : Ethylene reacts with ozone to form Ethylene ozonoid followed by the hydrolysis to form formaldehyde.
b) Reaction with KI : moist KI is oxidised to Iodine in presence of ozone.
2KI + H2O + O3 → 2KOH + I2 + O2
c) Reaction with Hg: Mercury loses it’s lustreness, meniscus and consequently sticks to the walls of glass vessel when it reacts with ozone. This phenomenon is called tailing of mercury.
2Hg + O3 → Hg2O + O2
It is removed by shaking it with water which dissolves Hg2O.
d) Reaction with PbS: Black lead sulphide oxidised to white lead sulphate in presence of ozone.
PbS + 4O3 → PbSO4 + 4O2.
![]()
Question 20.
a) What are the fuel cells? How are they different from Galvanic cells? Give the construction of H2 and O2 fuel cells.
b) What is molecularity of a reaction? How it is different from the order of a reaction? Name one bimolecular and one trimolecular gaseous reactions.
Answer:
(a) A fuel cell is a galvanic cell in which the chemical energy of fuel-oxidant system is converted directly into electrical energy.
- Conventional Galvanic cell converts chemical energy into electrical energy by spontaneous redox reactions.
- Fuel cell convert energy of combustion of fuels like hydrogen, methane etc., into electrical energy. These cause less pollution.
H3 – O2 fuel cell : In this cell, hydrogen and oxygen are bubbled through porous carbon electrodes into Cone. NaOH solution. Electrodes are embedded with suitable catalysts. The electrode reactions are :
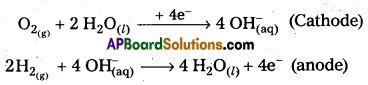
Overall reaction : 2H2(g) + O2(g)(g) → 2 H2O(l)
The cell functions as long as the reacting gases are in supply. The heat of combustion is directly converted into electrical energy.
(b) The number of reacting species (atoms, ions or molecules) taking parts in an elementary reaction, which must colloid Simultaneously to bring about a chemical reaction is called molecularity of a reaction.
NH4NO2 → N2 + 2H2O (Unimolecular)
2HI → H 2 + I2 (Bimolecular)
2NO + O2 → 2NO2 (Trimolecular)
Molecularity has only integer values (1, 2, 3 …….)
It has non zero, non fraction values while order has zero, 1, 2, 3 ……….. and fractional values.
It is determined by reaction mechanism, order is determined experimentally.
Question 21.
a) Explain the preparation of phenol from cumene,
b) Describe the following :
i) Cross aldol condensation
ii) Decarboxylation
Answer:
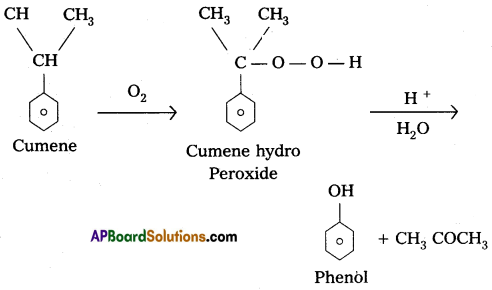
a) Preparation of phenol from cumene :
Cumene undergo reaction with O2 to forms cumene hydroperoxide followed by the hydrolysis to form phenol.
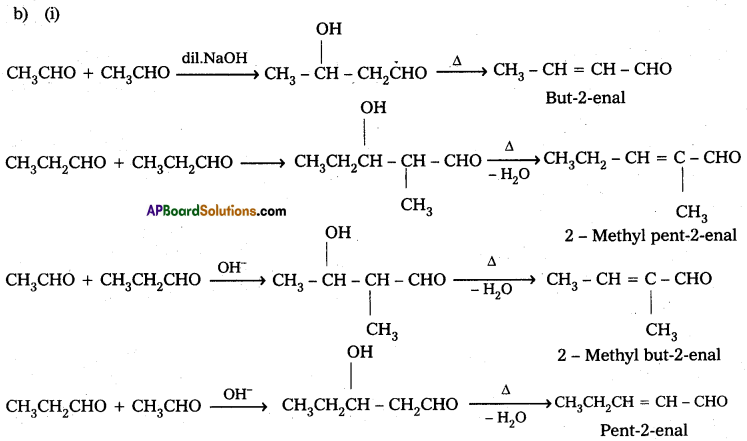
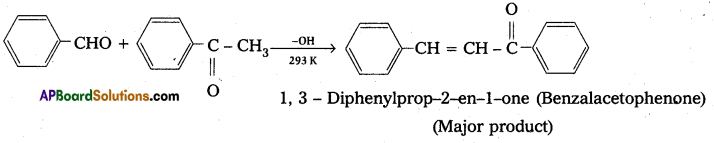
Ketones can also be used as one component in the cross aldol reactions
ii) Decarboxylation: Carboxylic acids lose carbon dioxide molecule to produce hydrocarbons on heating their sodium salts with sodalime (a mixutre of NaOH & CaO in ratio 3:1) This reaction is called decarboxylation