Access to a variety of TS Inter 1st Year Chemistry Model Papers and TS Inter 1st Year Chemistry Question Paper March 2019 helps students overcome exam anxiety by fostering familiarity.
TS Inter 1st Year Chemistry Question Paper March 2019
Note : Read the following instructions carefully.
- Answer all questions of Section ‘A’. Answer any six questions in Section ‘B’ and any two questions in Section ‘C’.
- In Section ‘A’, questions from Sr. Nos. 1 to 10 are of “Very Short Answer Type”. Each question carries two marks. Every answer may be limited to 2 or 3 sentences. Answer all these questions at one place in the same order.
- In Section ‘B’, questions from Sr. Nos. 11 to 18 are of “Short Answer Type”. Each question carries four marks. Every answer may be limited to 75 words.
- In Section ‘C’, questions from Sr. Nos. 19 to 21 are of “Long Answer Type”. Each question carries eight marks. Every answer may be limited to 300 words.
- Draw labelled diagrams wherever necessary for questions in Sections ‘3’ and ‘C’.
Section – A
Note : Answer all questions.
Question 1.
What is Bio-chemical Oxygen Demand (BOD)?
Answer:
The amount of oxygen used by the suitable micro organisms present in water during five days at 20° C is called as Bio Chemical Oxygen Demand (BOD).
Question 2.
What is a jBronsted base? Give an example.
Answer:
The substance which accepts a proton from the other substance is called Bronsted base e.g. : NH3, H2O etc.
![]()
Question 3.
Why is gypsum added to cement?
Answer:
Gypsum is added to cement to slow down the process of setting of the cement and to get sufficiently hardened cement.
Question 4.
Calculate the kinetic energy of 4 moles of methane at – 73°C.
Answer:

Question 5.
A solution is prepared by adding 2g of a substance ‘A’ to 18g of water, calculate the mass percentage of solute.
Answer:
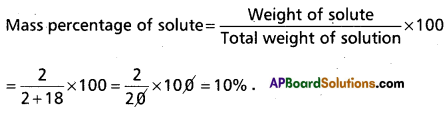
Question 6.
Write any two used of Mg metal.
Answer:
- Magnesium forms so many useful alloys with Al, Zn, Sn and
- Mg – Al alloys are useful in air – craft construction.
- Mg powder and ribbon is used in flash powders bulbs.
- Mg is used in incendiary bombs and signals.
- Milk of magnesice is used as antacid in medicine.
- MgCO3 is the main ingradient in tooth pastes.
Question 7.
Write the use of ZSM-5.
Answer:
- ZSM – 5 is a zeolite.
- It is used to convert alcohols directly into gasoline.
Question 8.
How does graphite function as a lubricant?
Answer:
Graphite is soft and it has layer lattice. In this structure one of the layer slided over another due to weak Vander Waal’s force of attraction. These layers are slippery. Hence it is greasy and function as lubricant.
Question 9.
Which oxides cause acid rain?
Answer:
Oxides of Nitrogen, Sulpihur and Carbon dissolved in rain water forms acid rain. Acid rain has pH value lessthan 5.6.
Question 10.
Write IUPAC names of the following structures :
Answer:
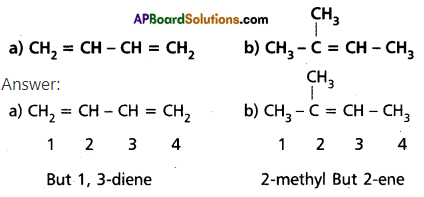
Question 11.
Derive an ideal gas equation.
Answer:
Ideal gas equation :
The combination of the gas laws leads to the development of an equation which relates to the four parameters volume, pressure, absolute temperature and number of moles. This equation is known as ideal gas equation. In this Boyle’s law and Charles’ law combined together and an equation obtained is called the gas equation.
Where V = volume of the gas
P = pressure of the gas
n = no. of moles of gas
T = absolute temperature
R = Universal gas constant.
Question 12.
A compound contains 4.07% Hydrogen, 24.27% Carbon and 71.65% Chlorine. Its molar mass is 98.96 g. What are its empirical and molecular formulas?
Answer:
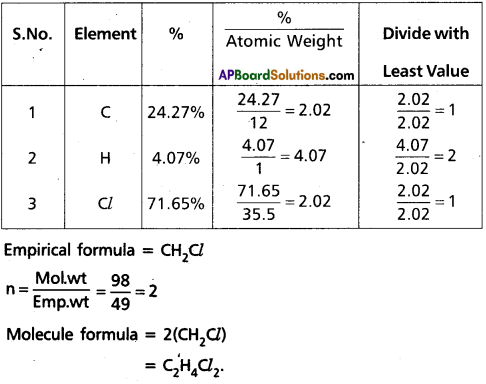
Empirical formula = CH2Cl
n = \(\frac{\text { Mol.wt }}{\text { Emp.wt }}\) = \(\frac{98}{49}\) = 2
Molecule formula = 2(CH2Cl)
= C2H4Cl2
![]()
Question 13.
State and explain the Hess’s law of constant heat summation.
Answer:
Hess’s Law: Hess’s law states that the total amount of heat evolved or absorbed in a chemical reaction is always same whether the reaction is carried out in one step (or) in several steps.
Illustration: This means that the heat of reaction depends only on the initial and final stages and not on the intermediate stages through which the reaction is carried out. Let us consider a reaction in which A gives D. The reaction is brought out in one step and let the heat of reaction be ∆H.
A → D; ∆H
Suppose the same reaction is brought out in three stages as follows
A → B : ∆H1
B → C : ∆H2
C → D : ∆H3
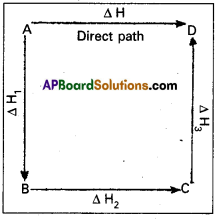
The net heat of reaction is ∆H1 + ∆H2+ ∆H3.
According to Hess law AH = ∆H1 + ∆H2 + ∆H3.
Ex : Consider the formation of CO2. It can be prepared in two ways.
1) Direct method : By heating carbon in excess of O2.
C(s) + O2(g) → CO2(g); ∆H = – 393.5 kJ
2) Indirect method : Carbon can be converted into CO2 in the following two steps.
C(s) + \(\frac{1}{2}\)O2(g) → CO2(g); ∆H1 = – 110.5 kJ
CO (g) + \(\frac{1}{2}\)O2(g) → CO2(g); ∆H2 = – 283.02 kJ
Total ∆H = -393.52 kJ
The two ∆H values are same.
Question 14.
Derive the relation between Kp and Kc for the equilibrium reaction.
N2(g) + 3H2(g) ⇌ 2NH3(g)
Answer:
N2(g) + 3H2(g) ⇌ 2NH3(g)
Relation between Kp and Kc is
Kp = Kc (RT)∆n
∆n = np – nR
= 2 – (1 + 3) = -2
∴ Kp < Kc
Question 15.
What is hard water? Write a note on the Calgon’s Method for the removal of hardness of water.
Answer:
- Hard water : Water does not give lather readily with soap is called hard water.
- Hard water contains hardness. This hardness is due to pres. ence of Ca, Mg soluble salts.
- Presence of Ca, Mg – bicarbonates causes temporary hardness.
- Presence of Ca, Mg – chlorides, sulphates causes permanent hardness.
ii) Calgon process:
- Calgon is sodium hexametaphosphate. [Na6P6O18 (or) (NaPO3)6]
- Calgon doesnot precipitate the Ca (or) Mg – salts but removes Ca+2 and Mg+2 ions from water.
- The removal of Ca+2 (or) Mg+2 ions from water may takes place either by adsorption (or) by complex formation.
Reactions:
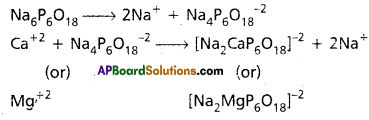
Question 16.
Explain Borax Bead Test with a suitable example.
Answer:
Borax bead test: This test is useful in the identification of basic radicals in qualitative analysis. On heating borax swells into a white, opaque mass of anhydrous sodium tetra borate. When it is fused, borax glass is obtained. Borax glass is sodium meta borate and B2O3. The boric anhydride, B2O3, combined with metal oxides to form metal metaborates as coloured beads. The reactions are as follows :
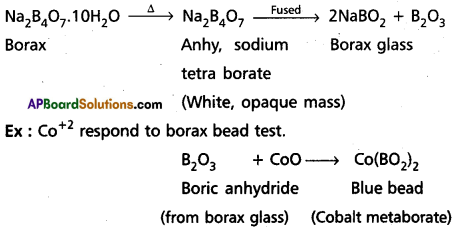
Question 17.
Explain the hybridization involved in SF6 molecule.
Answer:
In this hybridisation one ‘s’ orbital, three ‘p’ orbitals and two ‘d’ orbitals of the excited atom combine to form six equivalent sp3d2 hybrid orbitals.
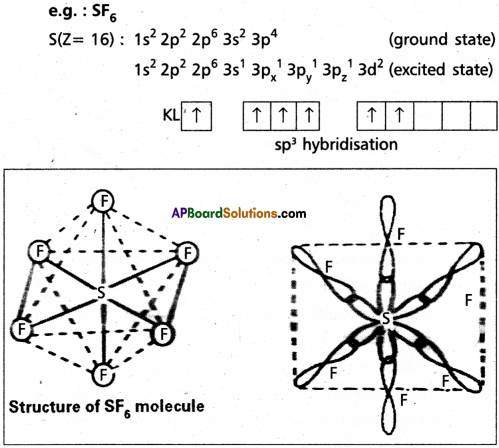
These six sp3 d2 hybrid orbitals overlap six 2pz orbitals of
fluorine atoms to form six asPV bonds. The directions of the bonds give an octa-hedra! shape to the molecule. The bond angle is 90° or 180° & 90°.
![]()
Question 18.
Give the Molecular Orbital Energy Diagram (MOED) of N2, Calculate the bond order of N2.
Answer:
Molecular orbital energy level diagram (MOED) of ‘N2‘:
Electronic configuration of nitrogen (z = 7) is 1s2 2s2 2p3. Since nitrogen atom has 7 electrons, the molecular orbitals of nitrogen molecule (N2) has 14 electrons which are distributed as below :
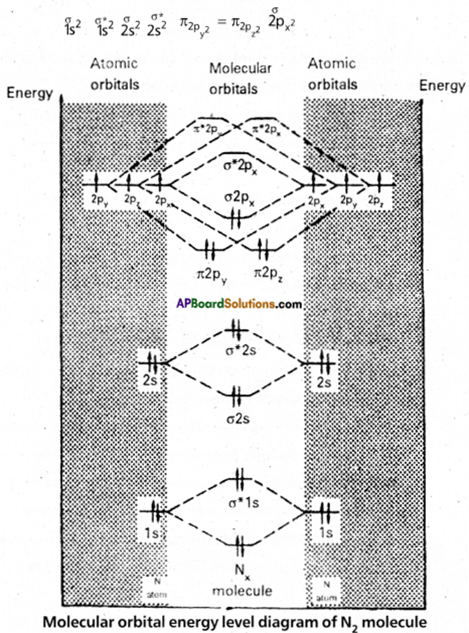
Bond order = \(\frac{8-2}{2}\) = 3 ( N = N)
Absence of unpaired electrons showed that N2 molecule is diamagnetic.
Question 19.
How are the quantum numbers n,l and m arrived at? Explain the significance of these quantum numbers.
Answer:
- In general a large no.of orbitals are posible in an atom.
- These orbitals are distinguished by their size, shape and ori-entation.
- An orbital of smaller size means there is more chance to find electron near the nucleus.
- Atomic orbitals are precisely distinguished by quantum num-bers. Each orbital is designated by three major quantum num-bers.
- Principal quantum number (n)
- Azimuthal quantum number (l)
- Magnetic quantum number (m)
1) Principal quantum number :
The principal quantum number was introduced by Neils Bohr. It reveals the size of the atom (main energy levels). With increase in the value of ‘n’ the distance between the nucleus and the orbit also increases. It is denoted by the letter ‘n’ It can have any simple integer value 1, 2, 3,….. but not zero. These are also termed as K, L, M, N etc.
The radius and energy of an orbit can be determined basing on “n” value.
The radius of nth orbit is rn = \(\frac{n^2 h^2}{4 \pi^2 m e^2}\)
The radius of nth orbit is En = \(\frac{-2 \pi^2 m e^4}{n^2 h^2}\)
2) Azimuthal quantum number:
It was proposed by Sommer- feld. It is also known as angular momentum quantum number or subsidiary quantum number. it indicates the shapes of orbitals. It is denoted by T. The values of T depend on the values of ‘n’, has values ranging from ‘0’ to (n – 1) i.e., I = 0, 1, 2,……. (n – 1). The maximum number of electrons present in the subshells s, p, d, f are 2, 6, 10, 14 respectively.
| Subshell | l – value | Shape |
| s | l = 0 | spherically symmetric |
| P | l = 1 | dumb – bell |
| d | l = 2 | double dumb-bell |
| f | l = 3 | four fold dumb-bell |
Energy levels and subshells
| Principal quantum number(n) | Azimuthal quantum number (l) | Symbol | Number of subshells |
| 1 | 0 | s | 1 (1s) |
| 2 | 0 | s | 2 (2s, 2p) |
| 1 | P | ||
| 3 | 0 | s | 3 (3s, 3p, 3d) |
| 1 | p | ||
| 2 | d | ||
| 4 | 0 | s | 4 (4s, 4p, 4d, 4f) |
| 1 | P | ||
| 2 | d | ||
| 3 | f |
3) Magnetic quantum number:
It was proposed by Lande. It shows the orientation of the orbitals in space, ‘p’ – orbital has three orientations. The orbital oriented along the x-axis is called pv orbital, along the y-axis is called px -orbital and along the py-axis is called pz orbital. In a similar way d – orbital has five orientations. They are dxy, dyz, dzx, dx2 – y2 and dz2. it is denoted by’m’. Its values depends on azimuthal quantum number, ‘m’ can have all the integral values from – l to +1 including zero. The total number of’m’ values are (21 +1).
| Subshell | lvalue | m value -1 to +l | No.of orbitals = 21 + 1 |
| s | 0 | 0 | 1 |
| P | 1 | -1,0, +1 | 3 |
| d | 2 | -2, -1,0, +1, +2 | 5 |
| f | 3 | -3,-2, -1,0, +1, +2, +3 | 7 |
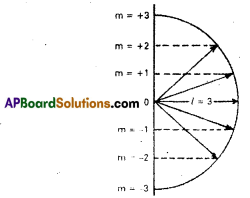
Question 20.
Define IE1 and IE2. Why IE2 > IE1 for the given atom? Discuss any four factors that affect IE of an element.
Answer:
1) Ionization energy is the amount of energy required to re¬move the most loosely held electron from isolated a neutral gaseous atom to convert it into gaseous ion. It is also known as first ionization energy because it is the energy required to remove the first electron from the atom.
It is denoted as I1 and is expressed in electron volts per atom, kilo calories (or) kilo joules per mole.
M(g) + I1 → M(g)+ + e–
l1 is first ionization potential.
2) The energy required to remove another electron from the unipositive ion is called the second ionization energy. It is denoted as I2.
M(g) + I2 → M(g)2+ + e–
3) The second ionization potential is greater than the first ionization potential. On removing an electron from an atom, the unipositive ion formed will have more effective nuclear charge than the number of electrons. As a result the effective nuclear charge increases over the outermost electrons. Hence more energy is required to remove the second electron. This shows that the second ionization potential is greater than the first ionization potential.
For sodium, I1 is 5:1 eV and I2 is 47.3 eV.
I1 < I2 < I3 ……. IN
Factors affecting ionization potential:
1. Atomic radius : As the size of the atom increases the distance between the nucleus and the outermost electrons increases. So the effective nuclear charge on the outermost electrons decreases. In such a case the energy required to remove the electrons also decreases. This shows that with an increase in atomic radius the ionization energy decreases.
2. Nuclear charge: As the positive charge of the nucleus increases its attraction increases over the electrons. So it becomes more difficult to remove the electrons. This shows that the ionization energy increases as the nuclear charge increases.
3. Screening effect or shielding effect : In multielectron atoms, valence electrons are attracted by the nucleus as well as
repelled by electrons of inner shells. The electrons present in the inner shells screen the electrons present in the outermost orbit from the nucleus. As the number of electrons in the inner orbits increases, the screening effect increases. This reduces the effective nuclear charge over the ojutermost electrons. It is called screening or shielding effect. With the increase of screening effect the ionization potential decreases. Screening efficiency of the orbitals falls off in the order s > p > d > f.
Magnitude of screening effect ∝ \(\frac{1}{\text { (Ionization enthalpy) }}\)
TREND IN A GROUP: The ionisation potential decreases in a group, gradually from top to bottom as the size of the elements increases down a group.
TREND IN A PERIOD: In a period from left to right I.P. value increases as the size of the elements decreases along the period.
![]()
Question 21.
Write any two methods of preparation of benzene with corre¬sponding equations. How methyl benzene and acetophenone are prepared from benzene.
Answer:
Preparation of Benzene:
1. When sodium benzoate distilled with sodalime. Benzene is formed.
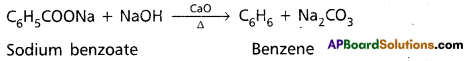
2. Polymerisation of Acetylene : When Acetylene gas is passed through red hot Cu or Fe tubes, it polymerises and give Benzene.
![]()
Constitution of Benzene : Benzene molecule cannot be represented by a single structure. It has the following Resonance structures.
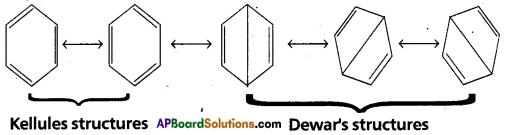
The resonance energy of Benzene is 150.48 KJ/mole. Thus, it has more stability. Hence does not undergo addition reactions like alkenes. Benzene is an aromatic molecule.
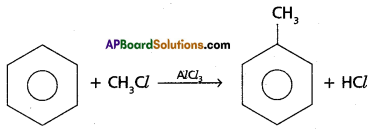
Benzene to toluene (Friedal Craft’s Alkylation):
Benzene reacts with methyl chloride in presence of AlC13 and forms methyl benzene (Toluene).
Preparation of Acetophenone from benzene:
Benzene reacts with acetyl chloride in presence of AlCl3 to from Acetophenone.
![]()