Access to a variety of TS Inter 1st Year Chemistry Model Papers and TS Inter 1st Year Chemistry Question Paper March 2018 helps students overcome exam anxiety by fostering familiarity.
TS Inter 1st Year Chemistry Question Paper March 2018
Note : Read the following instructions carefully.
- Answer all questions of Section ‘A’. Answer any six questions in Section ‘B’ and any two questions in Section ‘C’.
- In Section ‘A’, questions from Sr. Nos. 1 to 10 are of “Very Short Answer Type”. Each question carries two marks. Every answer may be limited to 2 or 3 sentences. Answer all these questions at one place in the same order.
- In Section ‘B’, questions from Sr. Nos. 11 to 18 are of “Short Answer Type”. Each question carries four marks. Every answer may be limited to 75 words.
- In Section ‘C’, questions from Sr. Nos. 19 to 21 are of “Long Answer Type”. Each question carries eight marks. Every answer may be limited to 300 words.
- Draw labelled diagrams wherever necessary for questions in Sections ‘3’ and ‘C’.
Section – A
Note : Answer all questions.
Question 1.
State first law of thermodynamics.
Answer:
First law of thermodynamics : The law of conservation of energy is taken as the first law of thermodynamics.
Statements :
- “Energy can neither be created nor destroyed, although it can be transformed from one form to another”.
- “It is impossible to construct perpetual motion machine of first kind”.
- “The total energy of the system and surroundings in constant”.
Question 2.
State Graham’s law of diffusion.
Answer:
The rate of diffusion of a given mass of gas at a given pressure and temperature is inversely proportional to the square root of its density. rate of diffusion r ∝ \(\frac{1}{\sqrt{d}}\)
![]()
Question 3.
Calculate the oxidation number of Manganese (Mn) in Mn04 ion.
Answer:
MnO4
x + 4(-2) = -1
x – 8 = -1
x = +7
Question 4.
What is Lewis acid ? Give one example.
Answer:
A substance which can accept an electron pair to from a coordinate covalent bond with donor is called Lewis acid. e.g. : H+, BF3, SnCl2 etc.
Question 5.
Lithium reacts with water less vigorously than sodium. Give reasons.
Answer:
Lithium reacts with water less vigorously than sodium.
Reasons :
- Lithium has small size.
- Lithium has very high hydration energy.
Question 6.
What happens when magnesium metal is burnt in air?
Answer:
Magnesium metal bums with dazzling brilliance in air to give MgO and Mg3N2.
2 Mg + O2 → 2 MgO; 3 Mg + N2 → Mg3N2
Question 7.
What is Biochemical Oxygen Demand (BOD)?
Answer:
The amount of oxygen used by the suitable micro organisms present in water during five days at 20° C is called as Bio Chemical Oxygen Demand (BOD).
Question 8.
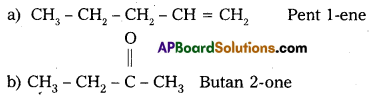
Write IUPAC names of the following structures :
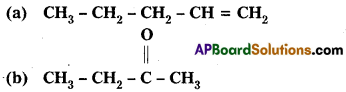
Answer:
Question 9.
Which gases cause Green House Effect ?
Answer:
Green house effect is caused by gases such as CO2, CH4, O3, CFCs (Chloro Fluoro Carbons) and water vapour in the atmosphere.
![]()
Question 10.
State Hess law of constant heat summation.
Answer:
Hess’s law states that the total amount of heat evolved or absorbed in a chemical reaction is always same whether the reaction is carried out in one step (or) in several steps.
Section – B
Question 11.
Write the postulates of kinetic molecular theory of gases.
Answer:
Assumptions :
- Gases are composed of minute particles called molecules. All the molecules of a gas are identical. ,
- Gaseous molecules are always, at a random movement. The molecules are moving in all possible directions in straight lines with very high velocities. They keep on colliding against each other and against the walls of the vessel at very small intervals of time.
- The actual volume occupied by the molecules is negligible when compared to the total volume occupied by the gas.
- There is no appreciable attraction or repulsion between the molecules.
- There is no loss of kinetic energy when the molecules collide with each other or with the wall of vessel. This is because the molecules are spherical and perfectly elastic in nature.
- The pressure exerted by the gas is due to the bombardment of the molecules of the gas on the walls of the vessel.
- The average kinetic energy of the molecules of the gas is directly proportional to the absolute temperature, Average K.E. M T.
- The force of gravity has no effect on the speed of gas molecules.
Question 12.
Balance the following equation in acid medium by Ion-electron method:
Fe(aq)+2 + Cr2O7(aq)2- fi Fe(aq)+3 + Cr(aq)+3
Answer:
H2O2 + Fe+2 → Fe+3 + H2O (Acidic solution)
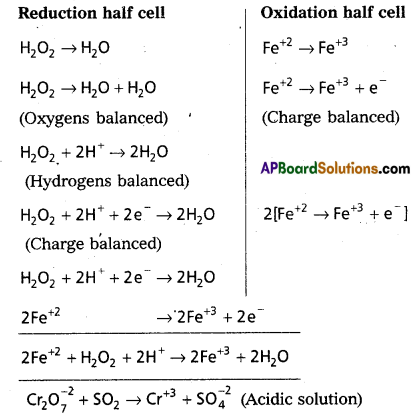
Question 13.
Explain hybridisation of phosphorous in the formation of PC15
Answer:
1) In PCl5 the electron configuration of phosphorus is
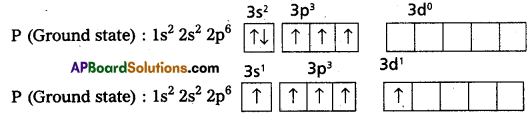
2) Phosphorus undergoes sp3d – hybridisation by intermixing of one s-orbital [3s], three p – orbitals [3px, 3py, 3pz] and one d – orbited.
These five hybrid orbitals overlap. The pz orbitals of chlorine atoms forming five σsp3d-s bonds. Out of these five p – Cl bonds three are coplanar and the remaining two are in the axial position. There by PCl5 acquires the trigonal bipyramidal shape. The molecule contains two bond angles 90° and 120°.
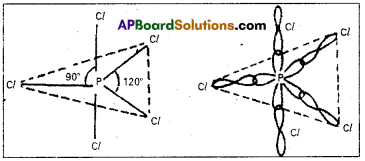
Question 14.
Discuss the application of Le-Chatlier’s principle for the industrial synthesis of Sulphur trioxide (SO3).
Answer:
Le-Chatlier’s Principle :
Statement: If a system at equilibrium is subjected to a change of P, T, concentration them the equilibrium shifts in such a direction in order to nullify the effect of change.
1. Effect of Pressure :
2 volumes of SO2 and one volume of O2 combine to give 2 volumes of SO3. According to Le Chatlier’s Principle increase of pressure favours the reaction where there is decrease in volume. The formation of SO3 is accompanied by decrease in volume (3 volumes to 2 volumes). Higher the pressure greater is the yield of SO3. But in contact process high pressures are not used because towers used in the manufacture are corroded by the acid at these high pressures. Low pressures favour the decomposition of SO3 as there is increase in the volume of (2 volumes to 3 volumes). Therefore optimum pressures are used (1.5 to 1.7 atmosphere).
2. Effect of Temperature : The formation of SO3 is exothermic. 189 kJ of heat is evolved. High temperatures favour the reverse reaction which is endothermic and do not favour the forward reaction which is exothermic. Low temperatures are favourable for the formation of SO3. At low temperature the reaction is too slow. Therefore an optimum temperature 673 K is used. To speed up the reaction V2Os is used as catalyst. Thus the optimum conditions are
Pressure – 1.5 to 1.7 atm
Temperature – 673 K
Catalyst – V2O5 (or) Platinised asbestos
![]()
Question 15.
What is Hydrogen bond? How many types? Give one example each.
Answer:
Hydrogen bond is a weak electrostatic bond formed between partially positive charged hydrogen atom and an highly electronegative atom of the same molecule or another molecule.
Hydrogen bond is formed when the Hydrogen is bonded to small, highly electronegative atoms like F, O and N. A partial positive charge will be on hydrogen atom and partial negative charge on the electronegative atom.
The bond dissociation energy of hydrogen bond is 40 KJ/ mole. Hydrogen bond is represented with dotted lines (…….). Hydrogen bond is stronger than Vander Waals’ forces and weaker than covalent bond. Hydrogen bonding is of two types. (1) Intermolecular hydrogen bond and (2) Intramolecular hydrogen bond.
1) Intermolecular hydrogen bond :
If the hydrogen bond is formed between two polar molecules it is called intermolecular hydrogen bond, i.e., the hydrogen bond is formed between hydrogen atom of one molecule and highly electronegative atom of another molecule is known as intermolecular hydrogen bond. Ex.: Water (H5O) ; HF : NH3 ; p – nitrophenol, CH3COOH, ethyl alcohol etc.
Water:
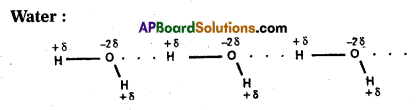
Water molecule forms an associated molecule through intermolecular hydrogen bond. Due to molecular association water possess high boiling point.
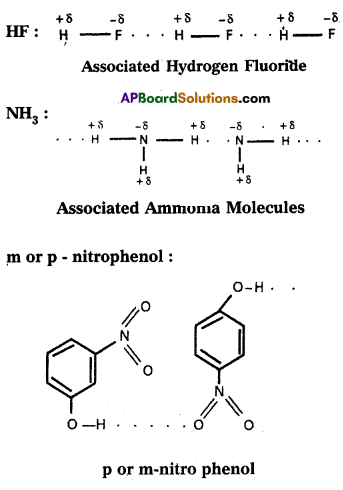
2) Intramolecular hydrogen bond :
If the hydrogen bond is formed within the molecule it is known as intramolecular hydrogen bond.
Ex.: o – nitrophenol; o – hydroxy benzaldehyde.
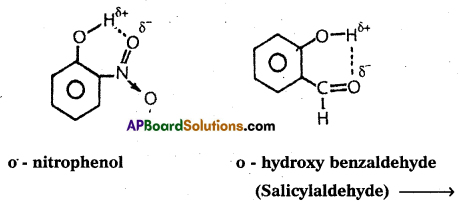
Question 16.
Write two, oxidation and two reduction reactions of Hydrogen peroxide.
Answer:
- H2O2 has the ability to function as an oxidising agent as well as reducing agent in both acid and alkaline solutions.
- In H2O2 oxidation state of oxygen is -1. It oxidised to O2.
Here H2O2 is reductant. - H2O2 can be reduced to H2O (or) OH–. Here H2O2 is oxidant.
i) Oxidising action in acidic medium :
2F(aq)+2 + 2H+(aq)) + H2O2(aq)
ii) Reducing action in acidic medium :
2MnO4– + 6H+ + 5H2O2 → 2Mn+2 + 8H2O + 5O2
(iii) Oxidising action in basic medium :
2Fe+2 + H2O2 → 2Fe+2 + 2OH–
iv) Reducing action in basic medium :
2MnO4– + 3H2O2 → 2MnO2 + 3O2 + 2H2O + 2OH–
Question 17.
Explain the structure of Diborane.
Answer:
Diborane is an electron deficient compound. It has ’12’ valency electrons for bonding purpose instead of 14′ electrons. In diborane each borane atom undergoes sp3 hybridization out of the four hybrid orbitals one is vacant.
Each borane forms two, a – bonds (2 centred – 2 electron bonds) bonds with two hydrogen atoms by overlapping with their ‘Is’ orbital. The remaining hybrid orbitals of boran used for the formation of B-H-B bridge bonds.
In the formation of B-H-B bridge, half filled sp3 hybrid orbital of one borane atom and vacant sp3 hybrid orbital of second borane atom overlap with Is orbital of H-atom.
These three centred two electron bonds are also called as banana bonds. These bonds are present above and below the plane of BH2 units. Diborane contains two coplanar BH2 groups. The four hydrogen atoms are called terminal hydrogen atoms and the remaining two hydrogens are called bridge hydrogen atoms.
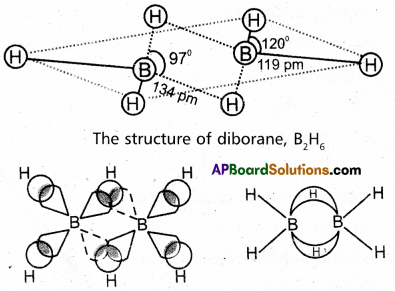
Bonding in diborane, Each B atom uses sp3 hybrids for bonding. Out of the four sp3 hybrids on each B atom, one is without an electron shown in broken lines. The terminal B-H bonds are normal 2-centre-2-electron bonds but the two bridge bonds are 3-centre-2-electron bonds. The 3-centre-2-electron bridge bonds are also referred to as banana bonds.
Question 18.
Give hybridisation of carbon in
(a) CO3-2
(b) diamond
(c) graphite
(d) fullerene
| a) CO3-2 | hybridisation |
| b) diamond | sp2 |
| c) graphite | sp3 |
| d) fullerene | sp2 |
Section – C
Question 19.
What are the postulates of Bohr’s model of hydrogen atom? Explain the formation of lines in the Hydrogen spectrum.
Answer:
Niels Bohr quantitatively gave the general features of hydrogen atom structure and it’s spectrum. His theory is used to evaluate several points in the atomic structure and spectra. The postulates of Bohr atomic model for hydrogen as follows.
Postulates :
- The electron in the hydrogen atom can revolve around the nucleus in a circular path of fixed radius and energy.
- These paths are called orbits (or) stationary states. These circular orbits are concentric (having same center) around the nucleus.
- The energy of an electron in the orbit does not change with time.
- When an electron moves from lower stationary state to higher stationary state absorption of energy takes place.
- When an electron moves from higher stationary state to lower stationary state emission of energy takes place.
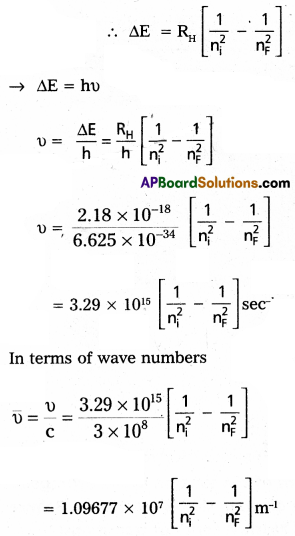
- When an electronic transition takes place between two stationary states that differ in energy by AE is given by . ΔE = E2 – E1 = hν
∴ The frequency of radiation absorbed (or) emitted ν =
\(\frac{E_2-E_1}{h}\) E1 and E2 are energies of lower, higher energy states respectively. - The angular momentum of an electron is given by mvr = \(\frac{\mathrm{nh}}{2 \pi}\)
An electron revolve only in the orbits for which it’s angular momentum is integral multiple of \(\frac{h}{2 \pi}\).
Line spectra of Hydrogen – Bohr’s Theory:
1. In case of hydrogen atom line spectrum is observed and this can be explained by using Bohr’s Theory.
2. According to Bohr’s postulate when an electronic transition takes place between two stationary states that differ in energy is given by
ΔE. = Ef – Ei
Ef = final orbit energy
Ei = initial orbit energy
We know that En =
3. In case of absorption spectrum nf > ni → energy is absorbed (+Ve).
4. In case of emission spectrum ni > nf → energy is emitted (-Ve).
5. Each spectral line in absorption (or) emission spectrum associated to the particular transition in hydrogen atom.
6. In case of large no.of hydrogen atoms large no.of transitions possible they results in large no.of spectral lines.
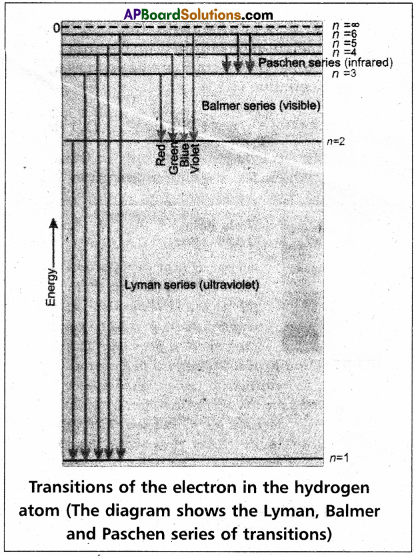
The series of lines observed in hydrogen spectra are
| ’n’ value | Series | Region |
| 1 | Lyman series | UV region |
| 2 | Balmer series | visible |
| 3 | Paschen series | Near I.R. |
| 4 | Brackette series | I.R. |
| 5 | Pfund series | Far I.R. |
Question 20.
How the following properties varies in a group and in a period ?
(a) Atomic radius
(b) Ionisation enthalpy
(c) Electronegativity-
(d) Electron gain enthalpy.
Answer:
Recurrence of similar properties of elements at definite regular intervals with increasing atomic number i.e., according to their electronic configurations is known as periodicity. Any prop¬erty which is periodic in nature is called periodic property.
a) Atomic radius:
The atomic radius decreases from left to right in a period. With an increase in the atomic number in a period the nuclear charge increases. As a result the effective nuclear charge over the outermost electrons increases, due to this the orbitals are pulled closer to the nucleus causing in a decrease in the atomic radius.
The atomic radius increases from top to the bottom in a group because – with an increase in the atomic number the electrons are added to new shells resulting an increase in the number of inner shells. Hence atomic radius increases from top to bottom in a group.
b) Ionisation enthalpy:
In groups and periods of the periodic table the ionization enthalpy values are periodically change de¬pend upon the electronic configuration and size of elements. In a group of elements ionization energy decreases from top to bottom because atomic radius increases. In general, in a period the atomic size decreases. Because of this, the ionization energy increases across a period.
c) Electronegativity:
Electronegativity increases from left to right in a period since the atomic: radii decreases and nuclear attraction increases. In a group electronegativity decreases from top to bottom due to an increase in atomic radii and a decrease in nuclear attraction.
d) Electron gain enthalpy:
Electron gain enthalpy increases in a period from left to right because the size of the atom decreases and the nature of the element changes from metallic to non – metallic nature when we move from left to right in a period. Electron gain enthalpy decreases from top to bottom in a group because there is an increase in the atomic size. But the second element has greater electron gain enthalpy than the first element.
e.g.: Chlorine has more electron affinity value (- 348 kJ mol-1) than Fluorine (- 333 kJ mol-1). It is because fluorine atom is smaller in size than chlorine atom. There is repulsion between the incoming electron and electrons already present in fluorine atom i.e., due to stronger inter electronic repulsions.
![]()
Question 21.
Write any two methods of preparation of ethylene. How does it reacts with the following?
(a) Cold, dil. alk. KMnO4
(b) Br2/CCl4
Answer:
Preparations of Ethylene :
1) From Ethyl Alcohol: Ethanol reacts with cone. H2SO4 at 170°C to form ethylene.
![]()
2) By dehydrohalogenation: Ethyl chloride reacts with alc.KOH to form ethylene.
C2H2a + alc.KOH → CH2 = CH2 + KCl + H2O
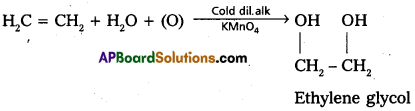
a) Cold, dil. alk. KMnO4: Pink coloured cold and dil. alkaline KMn04 solution is Bayer’s reagent. Ethylene decolourises Bayers reagent to give ethylene glycol. This is test for unsaturation.
b) With Bromine: Ethylene decolourises red coloured Br2 in CCl4 to give 1,2- dibromo ethane.
