Access to a variety of TS Inter 1st Year Chemistry Model Papers and TS Inter 1st Year Chemistry Question Paper March 2017 helps students overcome exam anxiety by fostering familiarity.
TS Inter 1st Year Chemistry Question Paper March 2017
Note : Read the following instructions carefully.
- Answer all questions of Section ‘A’. Answer any six questions in Section ‘B’ and any two questions in Section ‘C’.
- In Section ‘A’, questions from Sr. Nos. 1 to 10 are of “Very Short Answer Type”. Each question carries two marks. Every answer may be limited to 2 or 3 sentences. Answer all these questions at one place in the same order.
- In Section ‘B’, questions from Sr. Nos. 11 to 18 are of “Short Answer Type”. Each question carries four marks. Every answer may be limited to 75 words.
- In Section ‘C’, questions from Sr. Nos. 19 to 21 are of “Long Answer Type”. Each question carries eight marks. Every answer may be limited to 300 words.
- Draw labelled diagrams wherever necessary for questions in Sections ‘3’ and ‘C’.
Section – A
Note : Answer all questions.
Question 1.
Name two adverse effects caused by acid rains.
Answer:
Adverse effects caused by Acid rains :
- These cause respiratory ailments in human beings and animals.
- These damage the-old buildings and historical monuments like Tajmahal.
- These effect the plants and animal life in aquatic ecosystem.
Question 2.
What is Chemical Oxygen Demand (COD)?
Answer:
Chemical Oxygen Demand (COD) : The amount of oxygen required to oxidise the organic substances present in polluted water is called Chemical Oxygen Demand (COD).
![]()
Question 3.
Write the functional isomers of organic compound C3H6O.
Answer: Functional isomers of organic compound C3H60 are
CH3 – CH2 – CHO 1-propana
CH3 – CO – CH3 propan 2-one
Question 4.
Define inert pair effect.
Answer:
Inert pair effect: The reluctance of ‘ns1 pair of electrons to take part in bond formation is called inertpair effect. Ex : ‘Tl’ exhibits +1, oxidation state instead of +3 due to inert pair effect.
Question 5.
Write the biological importance of Na+ ions.
Answer:
Biological importance of Na+ ions :
- Na+ ions participate in the transmission of nerve signals.
- Na+ ions responsible or transport of sugars and aminoacids into cells.
- Na+ ions regulates the flow of water accross cell on embrAnswer:
Question 6.
What is meant by ionic product of water? What is its value at room temperature?
Answer:
Ionic product of water : The product of H+ ion concentration and OH– ion concentration in water is called ionic product of water (KW). The value of ionic product of water at room temperature is 1.008 × 10-14 mole2/lit2.
![]()
Question 7.
Calculate the kinetic energy of 5 moles of nitrogen at 27°C.
Answer:
KE = \(\frac{3}{2}\) nRT
n = 5 moles
R = 2 cal
T = 27° C = 27 + 273 = 300 K
= \(\frac{3}{2}\) × 5 × 2 × 300
= 4500 cal.
Question 8.
Calculate the oxidation number in Cr2O72- ion on chromium.
Answer:
Cr2O72-
2x + 7 (-2) = -2
2x – 14 = -2
2x = 12
x = +6
Question 9.
What is Piaster of Paris?
Answer:
The hemi hydrate of calcium sulphate is called plaster of paris,
The formula of plaster of pans is CaSO4\(\frac{1}{2}\)H2O.
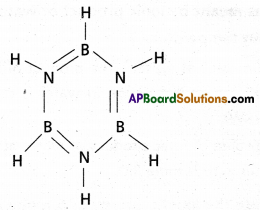
Question 10.
Give the formula of borazine. What is its common name?
Answer:
The formula of borazine is B3N3H6 (Borozole)
It’s common name is inorganic benzene. It is iso structural with benzene.
Section – B
Question 11.
Write the postulates of Kinetic Molecular Theory of Gases.
Answer:
Assumptions :
- Gases are composed of minute particles called molecules. All the molecules of a gas are identical.
- Gaseous molecules are always, at a random movement. The molecules are moving in all possible directions in straight lines with very high velocities. They keep on colliding against each other and against the walls of the vessel at very small intervals of time.
- The actual volume occupied by the molecules is negligible when compared to the total volume occupied by the gas.
- There is no appreciable attraction or repulsion between the molecules.
- There is no loss of kinetic energy when the molecules collide with each other or with the wall of vessel. This is because the molecules are spherical and perfectly elastic in nature. .
- The pressure exerted by the gas is due to the bombardment of the molecules of the gas on the walls of the vessel.
- The average kinetic energy of the molecules of the gas is directly proportional to the absolute temperature, Average K.E. ∝ T.
- The force of gravity has no effect on the speed of gas molecules.
Boyle’s law :
According to kinetic theory of gases, the pressure of a gas is due to collisions of gas molecules on the walls of the vessel. At a particular temperature the molecules make definite number of collisions with the walls of the vessel; When the volume of the vessel is reduced the molecules have to travel lesser distance only before making collisions on the walls. As a result the number of collisions per unit increases. The pressure then increases, i.e., the pressure increases when the volume is reduced at constant temperature. This explains Boyle’s law.
Charles’ law :
According to kinetic theory of gases, the average kinetic energy of the molecules is directly proportional to the absolute temperature of the gas.
K.E. ∝ T
but K.E. = \(\frac{1}{2}\) me2
As temperature increases, the velocity of the molecules also increases. As a result the molecules make more number of collisions against the walls of the vessel. This results in an increase of pressure if the volume is kept constant. If the volume is allowed to increase the number of collisions decrease due to the increased distance between the molecules and the walls of the vessel. The pressure then decreases. In other words, with rise of temperature, the volume should increase in order to keep the pressure constant. V ∝ T at constant pressure. This is Charles’ law.
![]()
Question 12.
A carbon compound contains 4.07% hydrogen, 24.27% carbon and 71.65% chlorine. Its molar mass is 98.06 gm. What are its empirical and molecular formulas?
Answer:
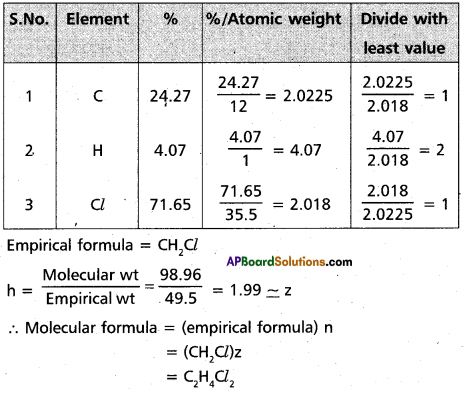
Question 13.
a) State the third law of thermodynamics.
Answer:
The entropy of a pure and perfectly crystalline substance is zero at absolute zero.
b) Define entropy.
Answer:
The measure of randomness or disorder ness of a system is called entropy (s).
![]()
Question 14.
Explain the concept of Bronsted-Lowry acid base theory with suitable examples.
Answer:
According to Bronsted theory a substance which can donate a proton to the other substance is known as acid. A substance which accept a proton from other substance is a base.
Ex : HCl + H2O ⇌ H3O+ + Cl2
NH3 + H2O ⇌ NH4+ + OH–
Here HCl donates a proton to water and behaves as Bronsted Lowry acid. Similarly NH3 accepts a proton from H2O and acts as Bronsted Lowry base.
Above reaction is a reversible reaction so that H3O+ can donate proton to act as acid. Cl-1 can accept a proton to act as base. Thus each acid base reaction equilibrium involves two acids and two bases. Each pair differs by a proton, such acid base pair is called Conjugate acid base ‘pair.
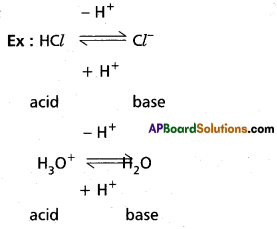
According to this theory strengths of acids and bases can be explained. An acid which show great tendency to donate protons is a strong acid and an acid which shows less tendency to donate proton is a weak acid. A base which shows great tendency to accept a proton is a strong base and a base which shows less tendency to accept a proton is a weak base.
Question 15.
a) Electron – deficient hydrides.
Answer:
These are the molecular hydrides in which the available no.of valency electrons is less than the number required for normal covalent bond formation.
(or)
These are the molecular hydrides in which the available valency electrons are lessthan the required for writting the Lewis structure of the molecule.
Eg : (AlH3)n, B2H6 etc.
These hydrides acts as Lewis acids i.e., electron pair acceptors. These forms dative bond with donors.
b) Electron – rich hydrides
Answer:
These are the molecular hydrides in which the valency electrons on the central atom are more than that are required for bond formation..
(or)
These are the molecular hydrides in which the available valency electrons are more than the required for writting the Lewis structure of the molecule.
- These hydrides contains lone pairs on central atoms.
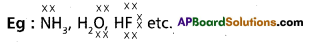
- These hydrides have high boiling points than those of the hydrides of the subsequent members of group because of hydrogen bond formation.
Question 16.
Explain the differences in properties of diamond and graphite on the basis of their structures.
Answer:
| Diamond | Graphite |
| a) Each carbon is sp3 hybri- dised. | a) Each carbon is sp2 hybri- dised. |
| b) Each carbon is bonded to 4 other carbons tetrahe- drally. | b) Each carbon is bonded to 3 other carbon atoms to form hexagonal rings. It has sheet like structure. |
| c) It has a 3 dimensional structure. | c) It has a 2 dimensional structure. |
| d) C – C bond length is 1.54 A° and bond angle is 109° 28′. | d) C – C bond length in hex- agonal rings is 1.42 A°and bond angle is 120°. |
| e) Carbon atoms are firmly held with strong covalent bonds. | e) The distance between two adjacent layers is 3.35 A°. These layers are held by weak Vander Waal’s forces. |
| f) Diamond is very hard. | f) Graphite is soft. |
| g) Density = 3.5 g/cc. | g) Density – 2.2g/cc. |
| h) Diamond is an insulator due to the absence of free electrons. | h) Graphite is a conductor due to the presence of free electrons. |
| i) It is transparent to light and X-rays. It has high refractive index (2.45). | i) It has layer lattice. The layers are slippery. Hence it is greasy. |
Question 17.
Explain Wurtz reaction and Friedel – Crafts alkylation with examples.
Answer:
Wurtz reactions:
Alkyl halides reacts with sodium metal in presence of dry ether to form alkanes.
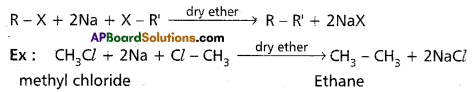
Friedal – Crafts alkylation:
Benzene reads with alkyl halides in presence of catalyst anhydrous AlC13 to from alkyl benzene.
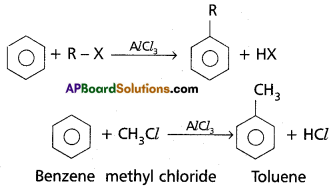
Question 18.
What is polymerization ? Explain with one example.
Answer:
Polymerization : A large molecular weight complex compound which is formed by the repeated combination of smaller units is called polymer. The process of formation of polymer is called polymerisation. Ex : Ethylene undergo polymerisation at 200°C and 1500 – 200 atm pressure gives polythene.

Section – C
Question 19.
a) What are the postulates of Bohr’s model of hydrogen atom?
Answer:
Postulates :
- The electron in the hydrogen atom can revolve around the nucleus in a circular path of fixed radius and energy. These paths are called orbits (or) stationary states. These circular orbits are concentric (having same center) around the nucleus.
- The energy of an electron in the orbit does not change with time. .
- When an electron moves from lower stationary state to higher stationary state absorption of energy takes place.
- When an electron moves from higher stationary state to lower . – stationary state emission of energy takes place.
- When an electronic transition takes place between two sta¬tionary states that differ in energy by ∆E is given by ∆E = E2 – E1 = hν)
∴ The frequency of radiation absorbed (or) emitted
ν = \(\frac{E_2-E_1}{h}\) E1 and E2 are energies of lower, higher energy states respectively. - The angular momentum of an electron is given by mvr = \(\frac{\mathrm{nh}}{2 \pi}\)
An electron revolve only in the orbits for which it’s angular momentum is integral multiple of \(\frac{\mathrm{h}}{2 \pi}\)
b) Explain the significance of ‘n’ and ‘l’ quantum numbers.
Answer:
1) Principal quantum number: The principal quantum number was introduced by Neils Bohr. It reveals the size of the atom (main energy levels). With increase in the value of ‘n’ the distance between the nucleus and the orbit also increases. It is denoted by the letter ‘n’. It can have any simple integer value 1, 2, 3, but not zero. These are also termed as K, L, M, N etc. The radius and energy of an orbit can be determined basing on “n” value.
The radius of nth orbit is rn = \(\frac{n^2 h^2}{4 \pi^2 m e^2}\)
The energy of nth orbit is En = \(\frac{-2 \pi^2 m e^4}{n^2 h^2}\)
2) Azimuthal quantum number :
It was proposed by Sommer- feld. It is also known as angular momentum quantum number or subsidiary quantum number. It indicates the shapes of orbitals. It is denoted by ‘l’. The values of ‘l’ depend on the values of ‘n’, T has values ranging from ‘O’ to (n – 1) i.e., l = 0, 1, 2,……(n – 1). The maximum number of electrons present in the subshells s, p, d, f are 2, 6, 10, 14 respectively.
| Subsheli | l- value | Shape |
| s | l = 0 | spherically symmetric |
| p | l = 1 | dumb – bell |
| d | l = 2 | double dumb-bell |
| f | l = 3 | four fold dumb-bell i |
Energy levels and subshelis
| Principal auanturn number (n) | Azimuthal quantum number (l) | Symbol | Number of subshells |
| 1 | 0 | s | 1 (1s) |
| 2 | 0 | s | 2 (2s, 2p) |
| 1 | p | ||
| 3 | 0 | s | 3 (3s, 3p, 3d) |
| 1 | p | ||
| 2 | d | ||
| 4 | 0 | s | 4 (4s, 4p, 4d, 4f) |
| 1 | p | ||
| 2 | d | ||
| 3 | f |
Question 20.
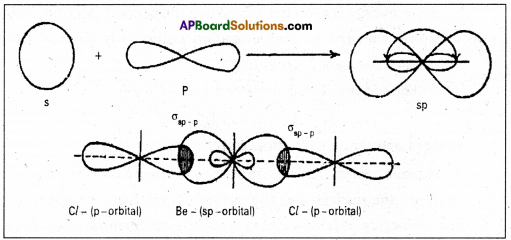
What is hybridization ? Explain sp, sp2 and sp3 hybridizations with one example each.
Answer:
Hybridisation is defined as the process of mixing of atomic orbitals of nearly equal energy of an atom to give the same number of new set of orbitals of equal energy and shapes.
Depending on the number and nature of orbitals involving hybridisation it is classified into different types. If ‘s’ and ‘p’ atomic orbitals are involved three types are possible namely sp3, sp2 and sp.
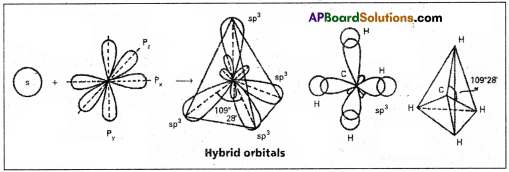
1. sp3 hybridisation :
In this hybridisation one’s and three ‘p‘ atomic orbitals of the excited atom combine to form four equivalent sp3 hybridised orbitals. This hybridisation is known as tetrahedral or tetragonal hybridisation.
Each sp3 hybridised orbital possess 25% ‘s’ nature and 75% of ‘p’ nature. The shape of the molecule is tetrahedral with a bond angle 109°28′, e.g. : Formation of Methane molecule :
- The central atom of methane is carbon.
- The electronic configuration of carbon in ground state is 1s2 2s2 2px2 2p20 2pz0 and on excitation it is 1s2 2s1 2px1 2py1 2pz1. During excitation the 2s pair splits and the electron jumps into the adjacent vacant 2pz orbital.
- The 2s1,2px1 2py1 2pz1 undergo sp3 hybridisation giving four equivalent sp3 hybridised orbitals.
- Each sp3 hybrid orbital overlaps with the 1s orbitals of hydrogen forming σGsp3-s bond.
- In case of methane four σGsp3-s bonds are formed. The bonds are directed towards the four corners of a regular tetrahedron. The shape of methane molecule is tetrahedral with a bond angle 109°28′.
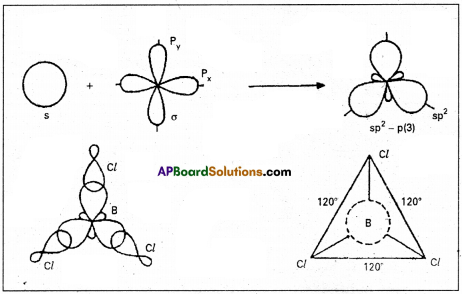
2. sp2 hybridisation :
In this hybridisation one ‘s’ and two ‘p’ atomic orbitals of the excited atom combine to form three equivalent sp2 hybridised orbitals. This hybridisation is also known as trigonal hybridisation. In sp2 hybridisation each sp2 hybrid orbital has 33.33% ‘s’ nature and 66.66% ‘p’ nature. The shape of the molecule is trigonal with a bond angle 120°.
E.g. : Boron trichloride molecule formation :
- The electronic configuration of ‘B’ in the ground state is 1s2 2s2 2px1 2py0 2pz0.
- On excitation the configuration is 1s2 2s1 2px1 2py1 2pz1. Now there are three half filled orbitals are available for hybridisation.
- Now sp2 hybridisation takes place at boron atom giving three sp2c hybrid orbitals.
- Each of them with one unpaired electron forms a ‘σ’ bond with one chlorine atom. The overlapping is σsp–-p (CZ atom has the unpaired electron in 2pz orbital). In boron trichloride there are three ‘σ’ bonds.
3. sp hybridisation :
In this hybridisation one ‘s’ and one ‘p’ atomic orbitals of the excited atom combine to form two equivalent sp hybridised orbitals. This hybridisation is also known as diagonal hybridisation, in sp hybridisation each sp hybrid orbital has 50% ‘s’ character and 50% ‘p’ character. The shape of the molecule is linear or diagonal with a bond angle 180°.
Ex. : Beryllium chloride molecule formation :
- Be atom has 1s2 2s2 2px0 2pb0 2pz0 electronic configuration.
- In ground state it has no half filled orbitals. On excitation the configuration becomes 1s2 2s1 2Px1 2py0 2pz0.
- Now sp hybridisation takes place at beryllium atom giving two sp hybrid orbitals. Each of them with one unpaired electron forms a ‘σ’ bond with one chlorine atom.
- The overlaping is σsp-p (Cl atom has the unpaired electron in 2pz orbital). In beryllium chloride there are two ‘σ’ bonds.
![]()
Question 21.
Define IE1 and IE2. Why is IE2 > IE1 for a given atom? Discuss the factors that affect IE of an element.
Answer:
1) Ionization energy is the amount of energy required to remove the most loosely held electron from isolated a neutral gaseous atom to convert it into gaseous ion. It is also known as first ionization energy because it is the energy required to remove the first electron from the atom. It is denoted as I1 and is expressed in electron volts per atom, kilo calories (or) kilo joules per mole.
M(g) + I1 → M(g)+ + e–
l1 is frist ionization potential.
2) The energy required to remove another electron from the unipositive ion is called the second ionization energy. It is denoted as I2.
M+(g) + l2 → M(g)2+ + e–
3) The second ionization potential is greater than the first ionization potential. On removing an electron from an atom, the unipositive ion formed will have more effective nuclear charge than the number of electrons. As a result the effective nuclear charge increases over the outermost electrons. Hence more energy is required to remove the second electron. This shows that the second ionization potential is greater than the first ionization potential. For sodium, I1 is 5.1 eV and I2 is 47.3 eV.
I1 < I2 < I3 …… In
Factors affecting ionization potential :
1. Atomic radius:
As the size of the atom increases the distance between the nucleus and the outermost electrons increases. So the effective nuclear charge on the outermost electrons decreases. In such a case the energy required to remove the electrons also decreases. This shows that with an increase in atomic radius the ionization energy decreases.
2. Nuclear charge :
As the positive charge of the nucleus increases its attraction increases over the electrons. So it becomes more difficult to remove the electrons. This shows that the ionization energy increases as the nuclear charge increases.
3. Screening effect or shielding effect:
In multielectron atoms, valence electrons are attracted by the nucleus as well as repelled by electrons of inner shells. The electrons present in the inner shells screen the electrons present in the outermost orbit from the nucleus. As the number of electrons in the inner orbits increases, the screening effect increases. This reduces the effective nuclear charge over the outermost electrons. It is called screening or shielding effect. With the increase of screening effect the ionization potential decreases. Screening efficiency of the orbitals falls off in the order s > p > d > f.
(Magnitude of screening effect) ∝ \(\frac{1}{\text { (Ionization enthalpy) }}\)
TREND IN’A GROUP :
The ionisation potential decreases in a group, gradually from top to bottom as the size of the elements increases down a group.
TREND IN A PERIOD :
In a period from left to right I.P. value increases as the size of the elements decreases along the period.