Access to a variety of TS Inter 1st Year Chemistry Model Papers and TS Inter 1st Year Chemistry Question Paper May 2016 helps students overcome exam anxiety by fostering familiarity.
TS Inter 1st Year Chemistry Question Paper May 2016
Note : Read the following instructions carefully.
- Answer all questions of Section ‘A’. Answer any six questions in Section ‘B’ and any two questions in Section ‘C’.
- In Section ‘A’, questions from Sr. Nos. 1 to 10 are of “Very Short Answer Type”. Each question carries two marks. Every answer may be limited to 2 or 3 sentences. Answer all these questions at one place in the same order.
- In Section ‘B’, questions from Sr. Nos. 11 to 18 are of “Short Answer Type”. Each question carries four marks. Every answer may be limited to 75 words.
- In Section ‘C’, questions from Sr. Nos. 19 to 21 are of “Long Answer Type”. Each question carries eight marks. Every answer may be limited to 300 words.
- Draw labelled diagrams wherever necessary for questions in Sections ‘3’ and ‘C’.
Section – A
Note : Answer all questions.
Question 1.
Define receptor and sink.
Answer:
Receptor: The medium which is effected by a pollutant is called receptor.
Sink : The medium which retains and interacts with long lived pollutant is called the sink.
Ex : Oceans are important sinks for atmospheric COr
Question 2.
Give the hybridisation of carbon in garphite and diamond.
Answer:
a) In CO3-2 carbon atom undergoes sp2 hybridisation.
b) In Diamond carbon atom undergoes sp3 hybridisation.
c) In Graphite carbon atom undergoes sp2 hybridisation.
d) In Fullerenes carbon atom undergoes sp2 hybridisation.
![]()
Question 3.
Calculate the oxidation numbers of oxygen in OF2 and O2 F2.
Answer:
OF2
x + 2(-1) = 0
x – 2 = 0
x = +2
O2 F2
2x + 2(-1) = 0
2x – 2 = 0
2x = 2
x = +1
Question 4.
Give any two biological importance of sodium and potassium ions.
Answer:
Biological importance of Na2 and K2 :
1. Na+ ions participate in transmission of nerve signals.
2. Na+ ions regulates the flow of water accross cell membrane.
3. K+ ions are useful in activating enzymes.
4. K+ ions participate in the oxidation of glucose to produce ATP.
Question 5.
Calculate the pH of 0.01 M HCl solution.
Answer: PH : The negative logarithm of H+ ion concentration base ten expressed in Moles/Lit.
PH = -log 10 [H+]
Problem:
Given 0.1 M HCI solution.
[H+] = 0.1 M
PH = – log[H+]
= -log 0.1
= – log 10-1
= 1
PH = 1
Question 6.
What is Chemical Oxygen Demand (COD)?
Answer:
Chemical oxygen demand (COD) : The amount of oxygen required to oxidise organic substance present in polluted water is called COD.
![]()
Question 7.
Which of the gas diffuses faster among N2,O2 and CH4 and why?
Answer:
CHH gas diffuse faster among N2, O2 and CH4.
Reason : CH4 (16) has low molecular weight than N2 (28) and O2 (32).
Question 8.
Write the formula of Plaster of Paris.
Answer:
Plaster of paris is the hemi hydrate of CaSO4 with formula
CaSO4.\(\frac{1}{2}\)H2O.
Preparation:
Plaster of paris is obtained by heating gypsum at 393 K.
![]()
- If temperature is used greater than 393 K then an hydrous CaSO4 is formed which is called ‘dead burnt plaster.
- Plaster of paris has an important property of setting with water.
- It forms a hard solid in 5 to 15 min. When it is mixed with suitable quantity of water.
- It is majorly used in building industry and as well as plasters.
- It is used in the bone fractures (or) sprain conditions.
- It is used in dentistry.
- It is used in manufacturing status and busts.
Question 9.
Write the IUPAC names of the following :
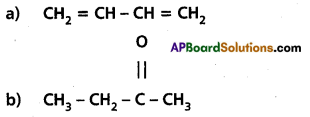
Answer:
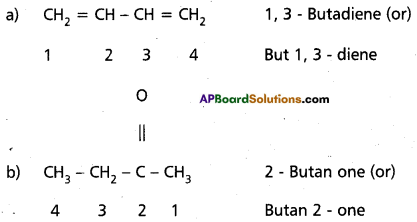
Question 10.
What is producer gas?
Answer:
- Producer gas is mixture of CO and N2.
- It is prepared by passing air over hot coke.
Section – B
Question 11.
State and explain the Hess’s law of constant heat summation with example.
Answer:
Hess’s law states that the total amount of heat evolved or absorbed in a chemical reaction is always same whether the reaction is carried out in one step (or) in several steps. Illustration : This means that the heat of reaction depends only on the initial and final stages and not on the intermediate stages through which the reaction is carried out. Let us consider a reaction in which A gives D. The reaction is brought out in one step and let the heat of reaction be ∆H.
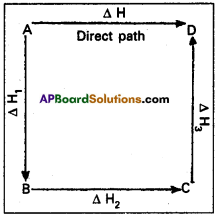
A → D; → ∆H
Suppose the same reaction is brought out in three stages as follows
A → B : ∆H1
B → C : ∆H2
C → D : ∆H3
The net heat of reaction is ∆H1 + ∆H2+ ∆H3.
According to Hess law ∆H = ∆H1 + ∆H1 + ∆H1.
Ex : Consider the formation of CO2. It can be prepared in two ways.
1) Direct method : By heating carbon in excess of O2
C(s) + O2(g) → CO2(g) ; ∆H = -393.5 kj
2) Indirect method : Carbon can be converted into CO2 in the following two steps.
C(s) + [/latex]\frac{1}{2}[/latex]O2(g) → CO2(g); ΔA1 = – 110.5 kj
CO(g) + [/latex]\frac{1}{2}[/latex]O2(g) → CO2(g); ΔA2 = – 283.02 kj
Total ΔH = -393.52 kj (∆H1 + ∆H2
The two ∆H values are same.
Question 12.
Explain the structure of Diborane.
Answer:
Diborane is an electron deficient compound. It has ’12’ valency electrons for bonding purpose instead of ’14’ electrons. In diborane each boron atom undergoes sp3 hybridization out of the four hybrid orbitals one is vacant. Each borane forms two a – bonds (2 centred – 2 electron bonds) bonds with two hydrogen atoms by overlapping with their’1s’orbital.
The remaining hybrid orbitals of boran used for the forma-tion of B-H-B bridge bonds. In the formation of B-H-B bridge, half filled sp3 hybrid orbital of one borane atom and vacant sp3 hybrid orbital of second boron atom overlap with 1s orbital of H-atom.
‘These three centred two electron bonds are also called as banana bonds. These bonds are present above and below the plane of BH2 units. Diborane contains two coplanar BH2 groups. The four hydro-gen atoms are called terminal hydrogen atoms and the remain-ing two hydrogens are called bridge hydrogen atoms.
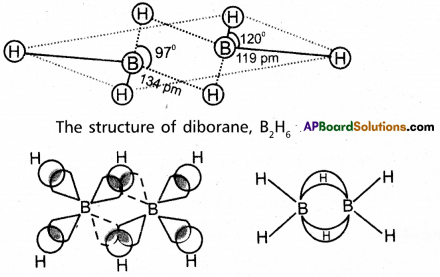
Bonding in diborane. Each B atom uses sp3 hybrids for bonding. Out of the four sp3 hybrids on each B atom, one is without an electron shown in broken lines. The terminal B-H bonds are normal 2-centre-2-electron bonds but the two bridge bonds are 3-centre-2-electron bonds. The 3-centre-2-electron bridge bonds are also referred to as banana bonds.
![]()
Question 13.
Define the terms hard water and soft water. How is temporary- hardness removed by Clark’s process?
Answer:
Hard water : Water does not give lather readily with soap is called hard water.
Hard water contains hardness. This hardness is due to presence of Ca, Mg soluble salts.
Presence of Ca, Mg – bicarbonates causes temporary hardness.
Presence of Ca, Mg – chlorides, sulphates causes permanent hardness.
Soft water : Water which give lather immediately with soap is called soft water.
(Or)
i) Ion – Exchange method :
- This method is useful to remove the permanent hardness of water.
- This method is also named as permutit (or) zeolite process.
- Permutit is the artificial zeolite, i.e. sodium aluminium orthosilicate. (Na2Al2Si2O2xH2O (or) NaAlSiO4)
- Permutit is written in short form as Naz.
- When permutit is added to hard water, the following ion- exchange reactions takes place.
2Naz(s) + Ca(aq)+2 → Caz2(s) + 2Na(aq)+
2Naz(s) + Mg(aq)+2 → Mg2(s) + 2Na(aq)+ - Caz2 and Mgz2 are called as exhausted permutit. These are regenerated to permutit by the treatment with brine solution. Caz(s) + 2Na(aq)+2 → 2Naz(s) + Ca(aq)+2
ii) Calgon process:
- Calgon is sodium hexametaphosphate. [Na6P6O18 (or) (NaPO3)6]
- Calgon doesnot precipitate the Ca (or) Mg – salts but removes Ca+2 and Mg+2 ions from water.
- The removal of Ca+2 (or) Mg+2 ions from water may takes place either by adsorption (or) by complex formation
Reactions:
Na6P6O18 → 2Na+ + Na4P6O18-2

Question 14.
Define Le Chatelier principle and mention the conditions for the preparation of the ammonia by Haber’s process.
Answer:
Lechatelier Principle Statement: When a system at equilibrium is subjected to a change of temperature, pressure, concentration then the equilibrium shifts in such a direction in order to nallify the effect of change.
Haber’s process:
N2(g) + 3H2(g) ⇌ 2NH3(g) + 92 kj
- In the above equilibrium recution decrease in no. of moles observed. So according to lechatelier’s principle high pressure favours the formation of NH3. So an optimum pressure of 200 atm is used.
- The given reaction is exothermic so low temperature favours the formation of NH3. But in industries at low temperature reaction proceds slowly. So suitable temperature is 725-775 K.
- To increase the rate of reaction catalyst ‘Fe’ is used and to increase the activity of catalyst promotor ‘Mo’ is used.
Pressure – 200 atm
Temperature – 725 – 775 k
Catalyst – Fe
Promotor – Mo
![]()
Question 15.
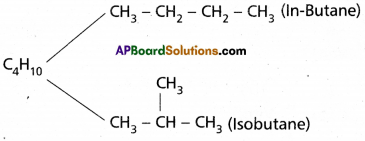
State and write chain and position isomerisms with examples.
Answer:
Chain Isomerism :
Compounds having same molecular formula but differ in the carbon chain or carbon skeleton are called chain isomer and this is called chain isomerism.
Eg:
Position Isomerism :
Compounds having same molecular formula but differ in the position of substituent or functional group or multiple bond are called position isomers and this is called position isomerism.
Eg:
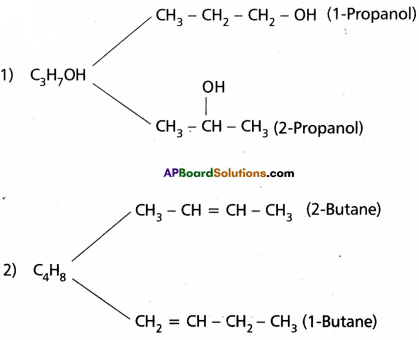
Question 16.
Define molarity. Calculate the molarity of NaOH in the solution prepared by dissolving 4 gr in enough water to form 250 ml of the solution.
Answer:
Molarity : The No.of moles of solute present in one liter of solution is called molarity (M).
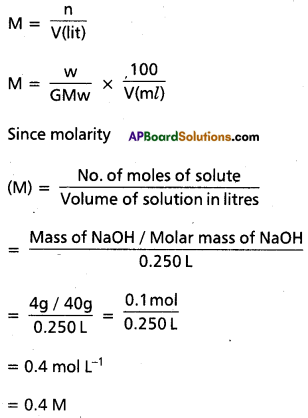
Note that molarity of a solution depends upon temperature because’volume of a solution is temperature dependent.
Question 17.
Complete the following reaction and name the products A, B and C:
![]()
Question 18.
Derive the Graham’s law of diffusion from kinetic gas equation.
Answer:
Derivation of Charle’s Law from Kinetic gas equation :
Kinetic gas equation is
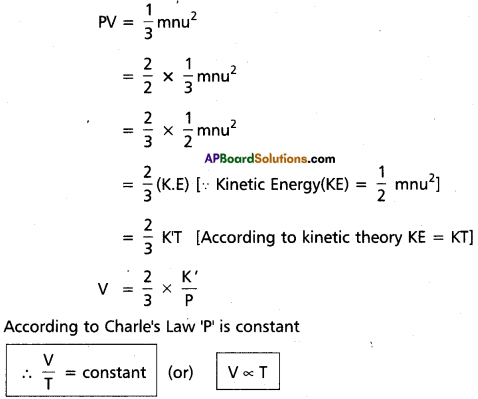
Hence Charle’s Law Proved from kinetic gas equation. Derivation of Graham’s Law of diffusion from Kinetic gas Equation. Knietic gas equation is
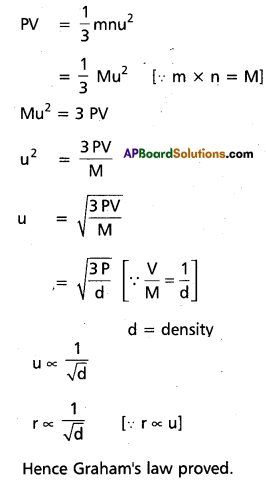
Section – C
Question 19.
Explain the significance of four quantum numbers associated with an electron in an atom.
Answer:
- In general a large no.of orbitals are possible in an atom.
- These orbitals are distinguished by their size, shape and orientation.
- An orbital of smaller size means there is more chance to find electron near the nucleus.
- Atomic orbitals are precisely distinguished by quantum numbers. Each orbital is designated by three major quantum numbers.
- Principal quantum number (n)
- Azimuthal quantum number (l)
- Magnetic quantum number (m)
1) Principal quantum number:
The principal quantum number was introduced by Neils Bohr. It reveals the size of the atom (main energy levels). With increase in the value of ‘n’ the dis¬tance between the nucleus and the orbit also increases.
It is denoted by the letter ‘n’. It can have any simple integer value 1, 2, 3,…… but not zero. These are also termed as K, L, M, N etc.
The radius and energy of an orbit can be determined basing on “n” value.
The radius of nth orbit is rn = \(\frac{n^2 h^2}{4 \pi^2 m e^2}\)
The radius of nth orbit is En = \(\frac{-2 \pi^2 m e^4}{n^2 h^2}\)
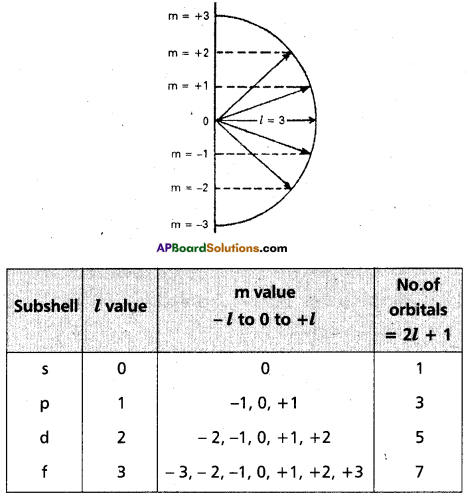
2) Azimuthal quantum number:
It was proposed by Sommer feld. It is also known as angular momentum quantum number or subsidiary quantum number. it indicates the shapes of orbitals. It is denoted by T. The values of T depend on the values of ‘n1, T has values ranging from ‘O’ to (n – 1) i.e., ( l = 0, 1, 2 …… (n – 1). The maximum number of electrons present in the subshells s, p, d, f are 2, 6, 10,14 respectively.
| Subshell | l – value | Shape |
| s | l = 0 | spherically symmetric |
| p | l = 1 | dumb – bell |
| d | l = 2 | double dumb – bell |
| f | l = 3 | four fold dumb – bell |
Energy levels and subshells
| Principal quantum number (n) | Azimuthal quantum number. (l) | Symbol | Number of subshells |
| 1 | 0 | s | 1 (1s) |
| 2 | 0 | s | 2 (2s, 2p) |
| 1 | P | ||
| 3 | 0 | s | 3 (3s, 3p, 3d) |
| 1 | p | ||
| 2 | d | ||
| 4 | 0 | s | 4 (4s, 4p, 4d, 4f) |
| 1 | P | ||
| 2 | d | ||
| 3 | f |
3) Magnetic quantum number:
It was proposed by Lande. It shows the orientation of the orbitals in space. ‘p’ – orbital has three orientations. The orbital oriented along the x – axis is called px orbital, along the py – axis is called pz – orbital and along the z – axis is called pz orbital. In a similar way d – orbital has five orientations. They are dxy
Question 20.
What is periodic property? How are the following properties vary in a group and period?
a) Atomic radius
b) Electron gain enthalpy
c) Electronegativity.
Answer:
Periodicity: The properties of elements which change gradually with change in electronic configuration and repeats in regular intervals are called periodic properties and this repetetion is called periodicity.
a) Atomic radius :
In groups and periods of the periodic table the ionization enthalpy values are periodically change depend upon the electronic configuration and size of elements. In a group of elements ionization energy decreases from top to bottom because atomic radius increases. In general, in a period the atomic size decreases. Because of this, the ionization energy increases across a period.
b) Electron gain enthalpy:
Electron gain enthalpy increases in a period from left to right because the size of the atom decreases and the nature of the element changes from metallic to non – metallic nature when we move from left to right in a period. Electron gain enthalpy decreases from top to bottom in a group because there is an increase in the atomic size. But the second element has greater electron gain enthalpy than the first element’.
e.g. : Chlorine has more electron affinity value ( – 348 kJ mol-1) than Fluorine (- 333 kJ mol-1). It is because fluorine atom is smaller in size than chlorine atom. There is repulsion between the incoming electron and electrons already present in fluorine atom i.e., due to stronger inter electronic repul, sions.
c) Electronegativity:
Electronegativity increases from left to right in a period since the atomic: radii decreases and nuclear attraction increases. In a group electronegativity decreases from top to bottom due to an increase in atomic radii and a decrease in nuclear attraction.
![]()
Question 21.
Define the hybridisation. Explain sp, sp2 and sp3 hybridisations each with one example.
Answer:
Hybridisation is defined as the process of mixing of atomic orbitals of nearly equal energy of an atom to give the same number of new set of orbitals of equal energy and shapes. Depending on the number and nature of orbitals involving hybridisation it is classified into different types. If ‘s’ and ‘p’ atomic orbitals are involved three types are possible namely sp3, sp2 and sp.
1. sp3 hybridisation:
In this hybridisation one’s and three ‘p’ atomic orbitals of the excited atom combine to form four equivalent sp3 hybridised orbitals.
This hybridisation is known as tetrahedral or tetragonal hybridisation. Each sp3 hybridised orbital possess 25% ‘s’ nature and 75% of ‘p’ nature. The shape of the molecule is tetrahedral with a bond angle 109°28′, e.g.: Formation of Methane molecule:
1) The central atom of methane is carbon.
2) The electronic configuration of carbon in ground state is 1s2 2s2 2p2x 2p1y 2p0z and on excitation it is 1s2 2s1 2p1x 2py1 2pz1. During excitation the 2s pair splits and the electron jumps into the adjacent vacant 2p2 orbital.
3) The 2s1 2p1x 2p1y 2p1z undergo sp3 hybridisation giving four equivalent sp3 hybridised orbitals.
4) Each sp3 hybrid orbital overlaps with the 1s orbitals of hydrogen forming σsp3-s bond.
5) In case of methane four σsp3-s bonds are formed. The bonds are directed towards the four corners of a regular tetrahedron. The shape of methane molecule is tetrahedral with a bond angle 109°28′.
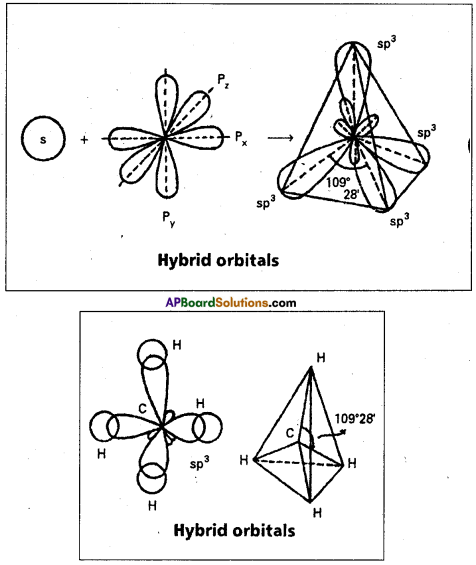
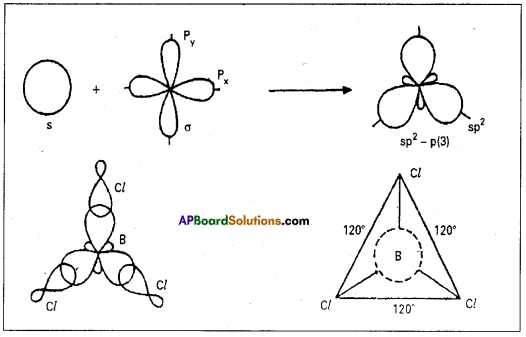
E.g.: Boron trichloride molecule formation :
1) The electronic configuration of ‘B’ in the ground state is 1s2 2s2 2p1x 2p0y 2p0z.
2) On excitation the configuration is 1s2 2s1 2p1x2p1y,2pz0 Now there are three half filled orbitals are available for hybridi-sation.
3) Now sp2 hybridisation takes place at boron atom giving three sp2 hybrid orbitals.
4) Each of them with one unpaired electron forms a ‘σ’ bond with one chlorine atom. The overlapping is σsp2-p (Cl atom has the unpaired electron in 2pz orbital). In boron trichloride there are three ‘σ’ bonds.
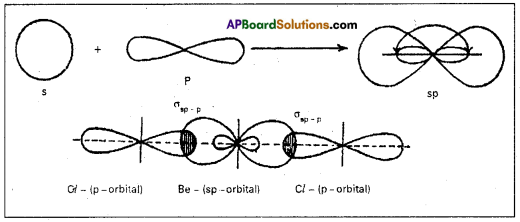
3. sp hybridisation:
In this hybridisation one ‘s’ and one ‘p’ atomic orbitals of the excited atom combine to form two equivalent sp hybridised orbitals.
This hybridisation is also known as diagonal hybridisation. In sp hybridisation each sp hybrid orbital has 50% ‘s’ character and 50% ‘p’ character. The shape of the molecule is linear or diagonal with a bond angle 180°.
Ex.: Beryllium chloride molecule formation :
1) Be atom has 1s1 2s1 2p0x 2p0y 2p0z electronic configuration.
2) In ground state it has no half filled orbitals. On excitation the configuration becomes 1s2 2s1 2p1x 2p0y2p0z
3) Now sp hybridisation takes place at beryllium atom giving two sp hybrid orbitals. Each of them with one unpaired electron forms a ‘o’ bond with one chlorine atom.
4) The overlaping is σsp-p (Cl atom has the unpaired electron in 2pz orbital). In beryllium chloride there are two ‘σ’ bonds.