Access to a variety of TS Inter 1st Year Chemistry Model Papers and TS Inter 1st Year Chemistry Question Paper May 2017 helps students overcome exam anxiety by fostering familiarity.
TS Inter 1st Year Chemistry Question Paper May 2017
Note : Read the following instructions carefully.
- Answer all questions of Section ‘A’. Answer any six questions in Section ‘B’ and any two questions in Section ‘C’.
- In Section ‘A’, questions from Sr. Nos. 1 to 10 are of “Very Short Answer Type”. Each question carries two marks. Every answer may be limited to 2 or 3 sentences. Answer all these questions at one place in the same order.
- In Section ‘B’, questions from Sr. Nos. 11 to 18 are of “Short Answer Type”. Each question carries four marks. Every answer may be limited to 75 words.
- In Section ‘C’, questions from Sr. Nos. 19 to 21 are of “Long Answer Type”. Each question carries eight marks. Every answer may be limited to 300 words.
- Draw labelled diagrams wherever necessary for questions in Sections ‘3’ and ‘C’.
Section – A
Note : Answer all questions.
Question 1.
Why is gypsum added to cement?
Answer:
Gypsum is added to cement to slow down the process of setting of the cement and to get sufficiently hardened cement.
Question 2.
Name any two man-made silicates.
Answer:
Cement and glass are man-made silicates.
Question 3.
What is Lewis acid? Give one example.
Answer:
Lewis acid: The substance which accepts electron pair and forms dative bond is called Lewis acid. E.g. :AlCl3, FeCl3, Ag+ etc.
Question 4.
Calcualte kinetic energy of 2 moles of Nitrogen at 27°C.
Answer:
Kinetic energy (KE) = \(\frac{3}{2}\) nRT
= \(\frac{3}{2}\) × 2 × 2 × 300 K
K = 1800 Cal.
![]()
Question 5.
Give an account of the biological importance of Na+ ions.
Answer:
Biological importance of Na+ :
- Na+ ions participate in the transmission of nerve signals.
- Na+ ions regulates the flow of water across cell membranes.
- Na+ ions responsible for transport of sugars and amino acids into cells.
Question 6.
Assign oxidation number to the underlined elements in
(a) NaHSO4
(b) KMnO4
Answer:
(a) NaHSO4
+ 1′ + 1 + x + 4(-2) = 0
2 + x – 8 = 0
x = + 6
(b) KMnO4
1 + x + 4(-2) = 0
1 + x – 8 = 0
x – 7 = 0
x = + 7
Question 7.
How does graphite function as a lubricant?
Answer:
Graphite is soft and it has layer lattice. In this structure one of the layer slided over another due to weak Vander Waal’s force of attraction. These layers are slippery. Hence it is greasy and function as lubricant. .
Question 8.
Give the possible BOD values of clean water and the polluted water.
Answer:
BOD value of pure water is 1 ppm. Fairly Pure water is 3 ppm and doubtful purity 5 ppm.
BOD of municipal sewage is 100 – 4000 ppm.
Question 9.
What is PAN? What effect is caused by it?
Answer:
- Peroxy Acetyl Nitrate is called as PAN.
- Peroxy Acetyl Nitrate is (PAN) is a powerful eye irritant.
![]()
Question 10.
Give two examples for position isomerism.
Answer:
Position isomerism : Compounds having same molecular formula but they differ in position of substituent or functional group (or) multiple bond are called position isomers. This is position isomerism.
Section – B
Question 11.
Explain the hybridization involved in SF6 molecule.
Answer:
In this hybridisation one ‘s’ orbital, three ‘p’ orbitals and two ‘d’ orbitals of the excited atom combine to form six equivalent sp3d2 hybrid orbitals, e.g. : SF6
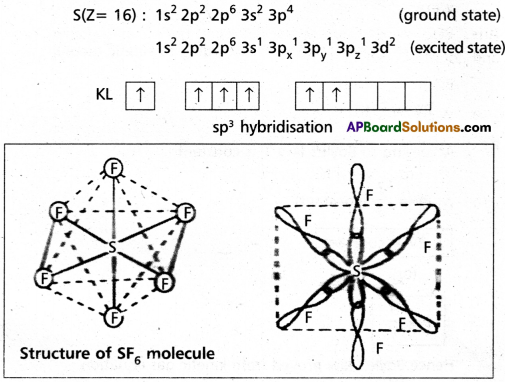
These six sp3d2 hybrid orbital six 2pz orbitals of fluoring atoms to form six σsp3d2 bonds. The directions of the bonds give an octa-hedral shape to the molecule. The bond angle is 90° or 180° & 90°.
Question 12.
Deduce (a) Boyle’s law and (b) Charle’s law from kinetic gas equation.
Answer:
a) Deduction of Boyle’s law :
Kinetic gas equation is
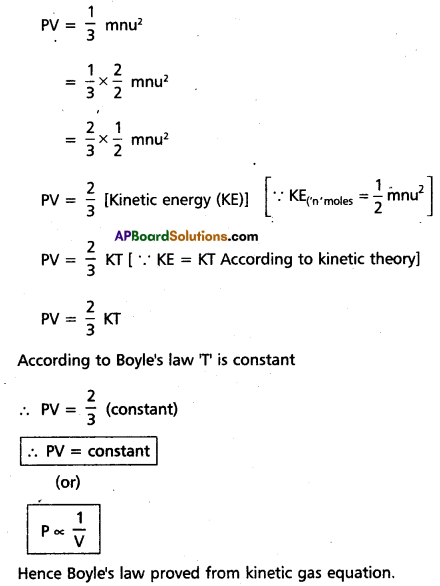
Hence Boyle’s law proved from kinetic gas equation.
b) Deduction of Charle’s law :
Kinetic gas equation is PV = \(\frac{1}{3}\) mnu2
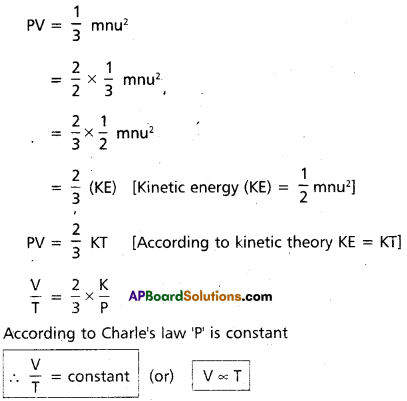
Hence Charle’s law proved from kinetic gas equation.
Question 13.
A carbon compound contains 12.8% carbon, 2.1 % Hydrogen, 85.1% Bromine. The molecular weight of the compound is 187.9. Calculate the molecular formula.
Answer:
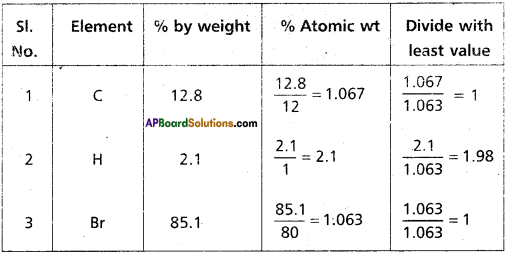
∴ Emperical formula of the compound = C1 H2 Br
Molecular formula = n (Emperical formula)
n = \(\frac{\text { Molecular wt }}{\text { Emperical wt }}=\frac{187.9}{94}=2\)
Given molecular wt = 187. 9
∴ Molecular formula = 2 (CH2Br)
= C2H4Br2
Given molecular wt = 187. 9
Emperical wt = 94 (CH4 Br)
![]()
Question 14.
State and explain the Hess’ law of constant heat summation.
Answer:
Hess’s Law :
Hess’s law states that the total arr\ount of heat evolved or absorbed in a chemical reaction is always same whether the reaction is carried out in one step (or) in several steps.
Illustration :
This means that the heat of reaction depends only on the initial and final stages and not on the intermediate stages through which the reaction is carried out. Let us consider a reaction in which A gives D. The reaction is brought out in one step and let the heat of reaction be ΔH.
A → D; ΔH
Suppose the same reaction is brought out in three stages as follows
A → B : ΔH1
B → C : ΔH2
C → D : ΔH3
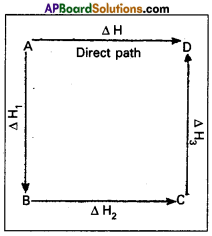
The net heat of reaction is ΔH1 + ΔH2+ ΔH3.
According to Hess law ΔH = ΔH1 + ΔH2 + ΔH3.
Ex : Consider the formation of CO2 It can be prepared in two ways.
1) Direct method: By heating carbon in excess of O2.
C(s) + O2(g) → CO2(g); ΔH = – 393.5 kJ
2) Indirect method: Carbon can be converted into CO2 in the following two steps.
C(s) + \(\frac{1}{2}\) O2(g) → CO2(g); ΔH1 = – 110.5 kJ
CO (g) + \(\frac{1}{2}\) O2(g) → CO2(g); ΔH2 = – 283.02 kJ
Total ΔH = -393.52 kJ
The two ΔH values are same.
Question 15.
Discuss the application of Le-Chatelier’s principle for the industrial synthesis of Ammonia.
Answer:
Applications of Le Chatelier’s principle to synthesis of Ammonia by Haber’s process :
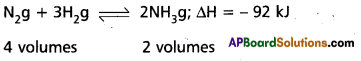
Nitrogen and Hydrogen combine to form ammonia. The formation of ammonia is reversible and exothermic reaction. It is accompanied by decrease in volume.
Effect of Pressure :
1 volume of N2 combines with 3 volumes of H2 to form 2 volumes of NH3. There is decrease in volume in the forward reaction (4 volumes to 2 volumes). According to Le Chatelier’s principle increase of pressure favours the reaction where there is decrease in volume. So higher the pressure, greater the yield of ammonia. In practice 200 atmospheres are used in the manufacture of ammonia by Haber’s process. Low pressures favour the reverse reaction i.e., decomposition of NH3 already formed.
Effect of Temperature : The formation of ammonia (forward reaction) is exothermic reaction
N2 + 3 H2 → 2 NH3; ΔH = -92 kj
Low temperatures favour the forward reaction. But at low tempera¬tures the reaction is too slow. Therefore an optimum temperature (725 K – 775 K) is chosen in Haber’s process. To speed up the reaction, a catalyst, finely divided iron is used. To increase the activity of the catalyst molybdenum or a mixture of oxides of K and M is used as promoter.
The reverse reaction (i.e.,) decomposition of NH3 is an endo¬thermic reaction. High temperatures favour the decomposition of NH3. Therefore high temperatures are avoided in Haber’s process.
Thus the optimum conditions are
Pressure : 200 atm
Temperature : 725 – 775 k
Catalyst : Fe (Powered)
Promoter : Mo (or) (K2O + Al2O3)
Question 16.
Write a few lines on the utility of Hydrogen as a fuel.
Answer:
Hydrogen as a fuel :
- The heat of combustion of hydrogen is high i.e about 242kj/ mole. Hence hydrogen is used as industrial fuel.
- The energy released by the combustion of dihydrogen is more , than the petrol (3 times).
- Hydrogen is major constituent in fuel gases like coal gas and water gas.
- Hydrogen is also used in fuel cells for the generation of electric power.
- 5% dihydrogen is used in CNG for running four-wheeler vehicles.
- By hydrogen economy principle the storage and transportation of energy in the form of liquid (or) gaseous state.
- Here energy is transmitted in the form of dihydrogen and not as electric power.
![]()
Question 17.
Explain borax bead test with a suitable example.
Answer:
Borax bead test: This test is useful in the identification of basic radicals in qualitative analysis. On heating borax swells into a white, opaque mass of anhydrous sodium tetra borate. When it is fused, bo¬rax glass is obtained. Borax glass is sodium meta borate and B2O3. The boric anhydride, B2O3, combined with metal oxides to form metal metaborates as coloured beads. The reactions are as follows :
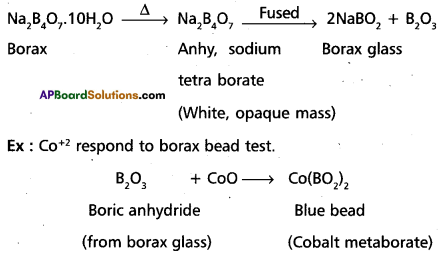
Question 18.
Explain the formation of co-ordinate covalent bond with one example.
Answer:
Co-ordinate covalent bond (dative bond) is a special type of co¬valent bond. It is proposed by Sidgwick. It is formed by the sharing of electrons between two atoms in which both the electrons of the shared electron pair are contributed by one atom and the other atom nearly participates in sharing. The bond is represented as (“→”) an arrow starting from the donar atom and directed towards the acceptor atom.
Exmples.
1) Ammonia – Boron trifluoride H3N : → BF3
Ammonia combines with boron trifluoride to give ammonium boron trifluoride.
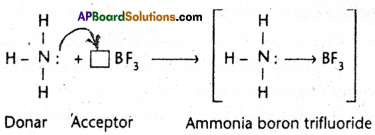
In ammonia nitrogen has a complete octet and also it has a lone pair of electrons. In BF3 the boron atom has a total of six electrons after sharing with fluorine. Nitrogen donates the electron pair to boron to form a co-ordinate covalent bond between ammonia and boron trifluoride.
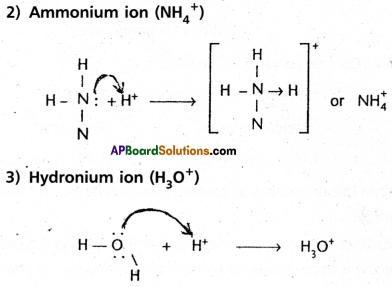
Properties of co-ordinate covalent bond :
- The bond do not ionise in water.
- The compounds are generally soluble in organic solvents and are sparingly soluble in water.
- These compounds exhibit space isomerism because the bond is rigid and directional.
- The bond is semipoiar in nature – so their volatility lies in between covalent and ionic bonds.
Section – C
Question 19.
How are the quantum numbers n, l and m arrived at? Explain the significance of these quantum numbers.
Answer:
- In general a large no.of orbitals are possible in an atom.
- These orbitals are distinguished by their size, shape and orientation.
- An orbital of smaller size means there is more chance to find electron near the nucleus.
- Atomic orbitals are precisely distinguished by quantum numbers. Each orbital is designated by three major quantum numbers.
- Principal quantum number (n)
- Azimuthal quantum number (l)
- Magnetic quantum number (m)
1) Principal quantum number :
The principal quantum number was introduced by Neils Bohr. It reveals the size of the atom (main energy levels). With increase in the value of ‘n’ the distance between the nucleus and the orbit also increases.
It is denoted by the letter ‘n’. It can have any simple integer value 1,2, 3, but not zero. These are also termed as K, L, M, N etc. The radius and energy of an orbit can be determined basing on “n” value.
The radius of n orbit is rn = \(\frac{n^2 h^2}{4 \pi^2 m e^2}\)
The radius of n orbit is En = \(\frac{-2 \pi^2 m e^4}{n^2 h^2}\)
2) Azimuthal quantum number: It was proposed by Sommerfeld. It is also known as angular momentum quantum number or subsidiary quantum number. It indicates the shapes of orbitals. It is denoted by T. The values of depend on the values of ‘n1, T has values ranging from ‘0’ to (n – 1) i.e., I = 0, 1, 2,……(n – 1). The maximum number of electrons present in the subshells s, p, d, f are 2, 6, 10, 14 respectively.
| Subshell | l value | Shape |
| s | 1 = O | spherically symmetric |
| P | 1 = 1 | dumb – bell |
| d | l = 2 | double dumb-bell |
| f | l = 3 | four fold dumb-bell |
Energy levels and subshells
| Principal quantum number(n) | Azimuthal quantum number(1) | Symbol | Number of subshells |
| 1 | 0 | s | 1 (1s) |
| 2 | 0 | s | 2 (2s, 2p) |
| 1 | P | ||
| 3 | 0 | s | 3 (3s, 3p, 3d) |
| 1 | p | ||
| 2 | d | ||
| 4 | 0 | s | 4 (4s, 4p, 4d, 4f) |
| 1 | P | ||
| 2 | d | ||
| 3 | f |
3) Magnetic quantum number:
It was proposed by Lande. It shows the orientation of the orbitals in space. ‘p’ orbital has three orientations. The orbital oriented along the x-axis is called px orbital, along the y-axis is called py – orbital and along the z-axis is called pz orbital. In a similar way d – orbital has five orientations. They are dxy , dyz, dzx, dx2-y2 and dz2 it is denoted by ‘m’. Its values depends on azimuthal quantum number, ‘m‘ can have all the integral values from -l to +1 including zero. The total number of ‘nT values are (21 + 1).
| Subshell | l value | m value -l to 0 to +l | No.orbitals = 2l + 1 |
| s | 0 | 0 | 1 |
| p | 1 | -1, 0, +1 | 3 |
| d | 2 | -2, -1,0, +1, +2 | 5 |
| f | 3 | -3, -2, -1, 0, +1, +2, +3 | 7 |
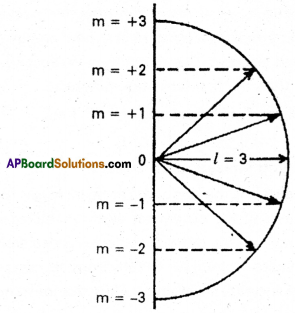
Question 20.
Define IE1 and IE2. Why is IE2 > IE1 for a given atom? Discuss four factors that effect IE of an element.
Answer:
1) Ionization energy is the amount of energy required to remove the most loosely held electron from isolated a neutral gaseous atom to convert it into gaseous ion. It is also known as first ionization energy because it is the energy required to remove the first electron from the atom.
It is denoted as I., and is expressed in electron volts per atom, kilo calories (or) kilo joules per mole.
M(g) + I1 → M(g)+ + e+
I1 is first ionization potential.
2) The energy required to remove another electron from the unipositive ion is called the second ionization energy. It is denoted as I2.
M+(g) + l2 → M(g)2+ + e–
3) The second ionization potential is greater than the first ionization potential. On removing an electron from an atom, the unipositive ion formed will have more effective nuclear charge than the number of electrons. As a result the effective nuclear charge increases over the outermost electrons. Hence more energy is required to remove the second electron. This shows that the second ionization potential is greater than the first ionization potential.
For sodium, I1 is 5.1 eV and I2 is 47.3 eV.
I1 is 5.1 ev and I2 is 47.3 eV
I1 < I2 < I3 …… In
Factors affecting ionization potential :
1) Atomic radius:
As the size of the atom increases the distance between the nucleus and the outermost electrons increases. So the effective nuclear charge on the outermost electrons decreases. In such a case the energy required to remove the electrons also decreases. This shows that with an increase in atomic radius the ionization energy decreases.
2) Nuclear charge:
As the positive charge of the nucleus increases its attraction increases over the electrons. So it becomes more difficult to remove the electrons. This shows that the ionization energy increases as the nuclear charge increases.
3) Screening effect or shielding effect:
In multi electron atoms, valence electrons are attracted by the nucleus as well as repelled by electrons of inner shells. Jhe electrons present in the inner shells screen the electrons present in the outermost orbit from the nucleus. As the number of’electrons in the inner orbits increases, the screening effect increases. This reduces the effective nuclear charge over the outermost electrons. It is called screening or shielding effect. With the increase of screening effect the ionization potential decreases. Screening efficiency of the orbitals falls off in the order s > p > d > f.
(Magnitude of screening effect) ∝ \(\frac{1}{\text { (Ionization enthalpy) }}\)
TREND IN A GROUP:
The ionisation potential decreases in a group, gradually from top to bottom as the size of the elements increases down a group.
TREND IN A PERIOD:
In a period from left to right I.P. value increases as the size of the elements decreases along the period.
![]()
Question 21.
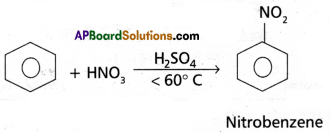
How do we get Benzene from acetylene? Give the corresponding. equation. Explain the halogenation, alkylation and acylation of benzene with equations.
Answer:
Preparation of Benzene from acetylene : On passing acetylene gas through red hot iron tubes, it trimerises to give benzene.
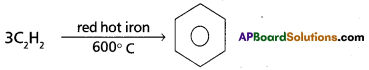
Chemical Properties:
(l)Halogenation: Benzene reacts with chlorine in the presence of FeCl3 or AlC3 to give chloro-benzene.
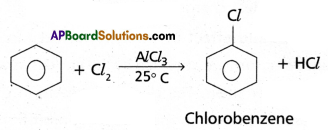
(2) ‘Friedel’- Craft’s Alkylation: Benzene reacts with alkyl halides in the presence of AlCl3 to give alkyl benzene.
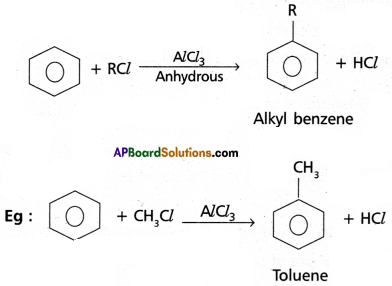
(3) Friedel – Craft’s Acylation: Benzene reacts with acetyl ‘chloride in the presence of AIC13 to give acetophenone.
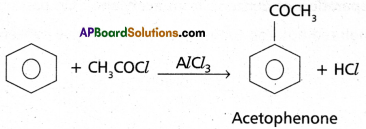
(4) Nitration : Benzene when heated with a mixture of cone. H2SO4 and cone. HNO3 below 60°C to give Nitrobenzene.
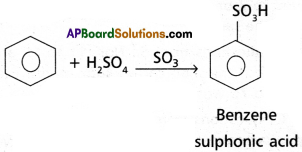
(5) Sulphonation : With fuming H2SO4, benzene, reacts to form benzene sulphonic acid