Timed practice with TS 10th Class Physical Science Model Papers Set 8 is crucial for improving speed and efficiency during exams.
TS 10th Class Physical Science Model Paper Set 8 with Solutions
Time: 1 Hour 30 minutes
Maximum Marks: 40
General Instructions:
- Read the question paper and understand every question thoroughly and write answers in given 1.30 hrs. time.
- 3 very short answer questions are there in section – I. Each question carries 2 marks. Answer all the questions. Write answer to each question in 3 to 4 sentences.
- 3 short answer questions are there in section – II. Each question carries 3 marks. Answer all the questions. Write answer to each question in 5 to 6 sentences.
- 3 essay type answer questions are there in section – III. Each question carries 5 marks. Answer all the questions. Write answer to each question in 8 to 10 sentences. Internal choice is given in this section.
Part – A (30 Marks)
Section – I (3 × 2 = 6 Marks)
Instructions :
- 3 Very short answer questions are there in section – I.
- Answer ALL the questions. Each question carries 2 marks.
- Write answer to each question in 3 to 4 sentences.
Question 1.
Write about various distances related to mirrors.
Answer:
The various distances related to mirrors are :
- Focal Length (f) : The distance between vertex and focus is called focal length.
- Radius of curvature (R) : The distance between vertex and centre of curvature is called radius of curvature.
- Object distance : It is the distance between the object and pole of mirror and is denoted by ‘u’.
- Image distance : It is the distance between the image and pole of the mirror and is denoted by ‘v’.
Question 2.
Why the least distance of distinct vision for children is 7 to 8 cm ?
Answer:
- In this age the muscles around the eye are strong and flexible and can bear more strain.
- So for children, the least distance of distinct vision is 7 to 8 cm.
Question 3.
Silicon is a metalloid. How do you support this ?
Answer:
Silicon exhibits following properties, so I conclude that it is a metalloid.
- It is metallic lustre in nature.
- It exists in several metallic and non-metallic compounds.
- It having brittle nature.
- All metalloids are usually occurs in combined states both metals and non-metals.
Section – II (3 × 3 = 9 Marks)
Instructions :
- 3 Short answer questions are there in section – II.
- Answer ALL the questions. Each question carries 3 marks.
- Write answer to each question in 5 to d sentences.
![]()
Question 4.
a) Which is more electro positive – Na or F ?
b) What is the number of electrons in the outer most orbit of Aluminium and Chlorine ?
c) Which forms cation easily – Mg or 0 ?
d) Which is better conductor of electricity – K or S ?
Answer:
a) Sodium (Z = 11) is more electro positive than F (Z = 9).
b) Al (Z = 13) : number of electrons present in outer most shell are = 3
Cl (Z = 17) : number of electrons present in outermost shell are = 7.
c) Magnesium (Z = 12) form cations easily than 0 (Z = 8).
d) Potassium (Z = 19) is better conductor of electricity than Sulphur (Z = 16).
Question 5.
Give electron dot formula for the following. ?
a) Magnesium chloride
b) Carbon dioxide
c) Carbon tetrachloride
d) Hydrogen bromide
Answer:
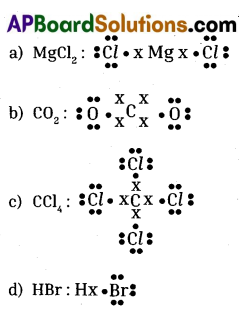
Question 6.
Which allotropes are used as good conductors ? Why ?
Answer:
1) Graphite and nanotubes are used as good conductors.
2) Both have layered structure. There is delocalised π electron system between layers. Because of this weaker system they are used as good conductors.
Section – III (3 × 5 = 15 Marks)
Instructions :
- 3 Essay answer questions are there in section.
- Answer ALL the questions. Each question carries 5 marks.
- Internal choice is given in this section.
- Write answer to each question in 8 to 10 sentences.
Question 7.
Explain the steps involved in balancing a chemical equation with an example.
(OR)
List out the material for the experiment to investigate whether all compounds containing Hydrogen are acids or not and write the experimental procedure.
Answer:
A chemical equation in which the number of atoms of different elements on the reactants side are same as those on product side is called a balanced equation.
Steps involved in balancing a chemical reaction : Let us consider the combustion reaction of Propane.
Step 1: Write the unbalanced equation using correct chemical formula for all substances.
C3H8 + O2 → CO2 + H2O (Skeleton equation)
Step 2 : Compare number of atoms of each element on both sides.
| Atom | No. of atoms in LH.S. | No. of atoms in RH.S. |
| C | 3 ((in C3 H3) | 1 (in CO2) |
| H | 8 (in C3 H3) | 2 (in H2O) |
| O | 2 (in O2) | 3 (inCO2, H2O) |
Find the co efficients to balance the equation. In this case, there are 3 carbon atoms on the left side of the equation but only one on the right side. If we add a co-efficient of 3 to CO2 on the right side the carbon atoms balance,
C3H8 + O2 → 3 CO2 + H2O
Now, look at the number of hydrogen atoms. There are 8 hydrogen atoms on the left but only 2 on the right side. By adding a co-efficient of 4 to the H2O on the right side, the hydrogen atoms get balanced.
C3H8 + O2 → 3 CO2 + 4 H2O
Finally, look at the number of oxygen atoms. There are 2 on the left side but 10 on the right side. By adding a co-efficient of 5 to the 02 on the left side, the oxygen atoms get balanced.
C3H8 + 5 O2 → 3 CO2 + 4 H2O
Step 3 : Make sure the co-efficients are reduced to their smallest whole number values. The above equation is already with the co efficients in smallest whole numbers. There is no need to reduce its co efficients. Hence the final equation is
C3H8 + 5 O2 → 3 CO2 + 4 H2O
Step 4 : Check the answer. Count the numbers and kinds of atoms on both sides of the equation to make sure they are the same.
(OR)
Procedure :
- Prepare glucose, alcohol, hydrochloric and sulphuric acid solution.
- Fix two iron graphite rods on a rubber cork and place the cork in a 100 ml beaker.
- Connect two different coloured electrical wires to graphite rods separately as shown in figure.
- Connect free ends of the wire to 230 volts AC plug.
- Complete the circuit as shown in the figure by connecting a bulb to one of the wires.
- Now pour some dilute HCl in the beaker and switch on the current.
Observation : The bulb starts glowing.
Repetition : Repeat activity with dilute sulphuric acid, glucose and alcohol solutions separately.
Observation :
- We will notice that the bulb glows only in acid solutions.
- But the bulb not glows in glucose and alcohol solutions.
Result :
- Glowing of bulb indicators that there is flow of electric current through the solution.
- Acid solutions have ions and the movement of these ions in solution helps for flow of electric current through the solution.
Conclusion :
- The positive ion (cation) present in HCl solution is H+.
- This suggests that acids produced hydrogen ions H+ in solution, which are responsible for their acidic properties.
- In glucose and alcohol solutions the bulb did not glow indicating the absence of H+ ions in these solutions.
- The acidity of acids is attributed to the H+ ions produced by them in solutions.
![]()
Question 8.
Write the characteristics of the images formed by a convex lens having focal length of 25 cm, when an object is kept on the principle axis at a distance of 50 cm and 75 cm.
(OR)
Write postulates and limitations of Bohr Hydrogen atomic model.
Answer:
When the object is kept at 50 cm in front of the lens, the characteristics of image :
- Image will form at 50 cm distance.
- Size of image is equal to the size of the object.
- Image is inverted.
- Image is real.
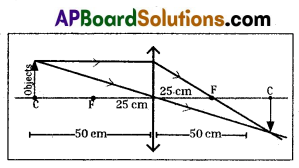
When the object is kept at 75 cm in front of the lens, the characteristics of image:
- Image will be formed between F and C (app. 37.5 cm)
- Diminished image will be formed.
- Image is inverted.
- Image is real.
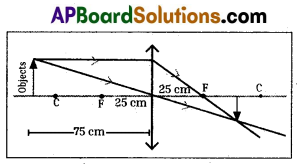
(OR)
Postulates :
- Electrons revolve around nucleus in stationary circular orbits of fixed energies which are called energy levels.
- As long as electron revolves in a stationary orbit, it neither loses nor gains energy.
- These stationary orbits are denoted by the letters K, L, M, N …. or by the number n = 1, 2, 3, 4, ………….. , where, n stands for orbit number.
Limitations :
- Bohr failed to explain atomic spectra of larger atoms which are heavier than hydrogen atom.
- He also unable to explain the relative intensities of spectral lines, the existence of hyperfine lines and the Zeeman effect.
Question 9.
In a circuit, 60 V battery, three resistances R1 = 10Ω, R2 = 20Ω and R3 = x Ω are connected in series. If 1 ampere current flows in the circuit, find the resistance R3 by using Kirchhoff’s Loop law.
(OR)
A circular coil of radius 10 cm, 500 turns and resistance 2Ω is placed with its plane perpendicular to the horizontal component of the earth’s magnetic field. It is rotated about its vertical diameter through 180° in 0.25 sec. Estimate the magnitudes of the EMF and current induced in the coil. (Horizontal component of the earth’s magnetic field at the place is 3.0 × 10-5T)
Answer:
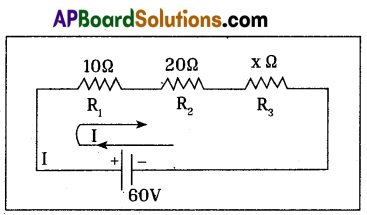
According to Loop law,
60 – 10(I) – 20(I) – x(I) = 0
Substituting I = 1 Amp, in the above equation,
60 – 10 – 20 – x = 0
x = 30 Ω
∴ R3 = 30 Ω
Another method :
R1 = 10 Ω, R2 = 2o Ω, R3 = x Ω
As they are connected in series, the resultant
Resistance R = R1 + R2 + R3
= 10 Ω + 20 Ω + 1 Ω
= 30 + x Ω
I = 1 Amp, V = 60 V
According to Ohm’s law.
V = IR
60 V = 1 × (30 + x Ω)
60 V = 30 + x Ω
x = 60 – 30
x = 30 Ω
∴ R3 = 30 Ω.
(OR)
Earth’s magnetic field ‘B’= 3.0 × 10-5 T
Area of the coil = πr2 = π × 10-2 m2.
(radius = 10 cm = 10-1m)
Initial flux through the coil
Q(initial) = BA cos θ
= (3.0 × 10-5) × (π × 10-2) × cos 180° = 3π × 10-7 wb
(since the plane of coil is perpendicular to the magnetic field, the angle is zero)
Final flux after rotation
Q(initial) = BA cos θ = 3.0 × 10-5 × π × 10-2 × cos 180°
= – 3π × 10-7wb
Total flux = (3π × 10-7) – (- 3π × 10-7)
= 6π × 10-7 wb
The value of estimated emf in
ε = N.\(\frac{\Delta \phi}{\Delta t}\) = 500 × \(\frac{6 \pi \times 10^{-7}}{0.25}\) = 3.8 × 10-3V
The value of estimated current is
I = \(\frac{3: 8 \times 10^{-3}}{2 \Omega}\) = 1.9 × 10-3A.
![]()
Part – B (10 × 1 = 10 Marks)
Instructions :
- Answer ALL the questions.
- Each question carries 1 mark.
- In this section there are 4 options (A / B / C / D) to each question. Choose the appropriate answer and write the answer in the brackets given against the question. Part – B must be attached to the answer booklet of Part – A.
Question 1.
When parallel rays are Incident on a concave mirror, on reflection they meet at the
A) focus
B) pole
C) centre of curvature
D) none of these
Answer:
A) focus
Question 2.
If some amount of energy is released in a chemical reaction, then It Is called ……….. reaction.
A) exothermic
B) endothermic
C) oxidation
D) reduction
Answer:
A) exothermic
Question 3.
Why is universal indicator a better one than litmus paper ?
A) Litmus paper can only be used for acids.
B) Litmus paper can only be used for alkalies.
C) Universal indicator goes green if some thing is neutral.
D) Universal indicator is useful for all ranges of pH of the solution.
Answer:
D) Universal indicator is useful for all ranges of pH of the solution.
Question 4.
Tooth decay starts when the pH of the mouth is
A) 5.5
B) greater than 5.5
C) lower than 5.5
D) all of these
Answer:
C) lower than 5.5
Question 5.
Which of the following is the chemical formula for hydrated copper sulphate ?
A) CuSO4.6H2O
B) CuSO4.7H2O
C) CuSO4.2H2O
D) CUSO4.5H2O
Answer:
D) CUSO4.5H2O
Question 6.
If the convex lens is placed in water its focal length is ……………….
A) Increases
B) Decreases
C) remains constant
D) either increase or decrease
Answer:
A) Increases
![]()
Question 7.
If the refracted ray from a convex lens is travelling parallel to the principal axis, then image distance is
A) Equal to object distance.
B) Infinity
C) Equal to focal length of the lens
D) Equal to radius of curvature of the lens
Answer:
B) Infinity
Question 8.
In which type of defect in vision that people cannot see objects at long distances but can see nearby objects clearly ?
A) hypermetropia
B) presbyopia
C) myopia
D) all
Answer:
B) presbyopia
Question 9.
Which of the following indicatesThe correct order of variation in atomic size?
A) Be < C < F < Ne
B) Be > C < F < Ne
C) Be > C > F > Ne
D) F < Ne < Be < C
Answer:
B) Be > C < F < Ne
Question 10.
The reducing agent in thermite process is
A) Al
B) Mg
C) Fe
D) Si
Answer:
A) Al