Timed practice with TS 10th Class Physical Science Model Papers Set 6 is crucial for improving speed and efficiency during exams.
TS 10th Class Physical Science Model Paper Set 6 with Solutions
Time: 1 Hour 30 minutes
Maximum Marks: 40
General Instructions:
- Read the question paper and understand every question thoroughly and write answers in given 1.30 hrs. time.
- 3 very short answer questions are there in section – I. Each question carries 2 marks. Answer all the questions. Write answer to each question in 3 to 4 sentences.
- 3 short answer questions are there in section – II. Each question carries 3 marks. Answer all the questions. Write answer to each question in 5 to 6 sentences.
- 3 essay type answer questions are there in section – III. Each question carries 5 marks. Answer all the questions. Write answer to each question in 8 to 10 sentences. Internal choice is given in this section.
Part – A (30 Marks)
Section – I (3 × 2 = 6 Marks)
Instructions :
- 3 Very short answer questions are there in section – I.
- Answer ALL the questions. Each question carries 2 marks.
- Write answer to each question in 3 to 4 sentences.
Question 1.
Identify types of mirrors without actually touching it. (move the mirror to and from)
Answer:
- Plane mirror: It forms the image is of same size.
- Concave mirror: Image is curved, bringing the mirror closer, magnifies the image. Moving it away, the image is inverted and reduced.
- Convex mirror: Mirror image is always diminished but erect. The view point is wider.
Question 2.
Why do presbyopia is seen in aged people ?
Answer:
- Presbyopia is generally seen in aged people.
- This is due to gradual weakening of ciliary muscles and diminishing flexibility of the eye lens.
- So that the ability of accomodation of the eye gradually, decreases with ageing.
Question 3.
Which ores of metals are needed roasting during extraction process ? Why ?
Answer:
- The ores of such metals like zinc, iron, tin, lead and copper need roasting process.
- Because they generally present as sulphides or Carbonates in nature! So first they converted into oxides by heating in excess air. This is nothing but roasting.
- Then these, metal oxides are reduced into their metals.
![]()
Section – II (3 × 3 = 9 Marks)
Instructions :
- 3 Short answer questions are there in section – II.
- Answer ALL the questions. Each question carries 3 marks.
- Write answer to each question in 5 to 6 Sentences.
Question 4.
Explain Newland’s law of octaves with an example.
Answer:
- Newlands arranged the elements in the ascending order of their atomic weights.
- According to Newlands, if we start with hydrogen in the table, the eighth element is fluorine and next eighth element is chlorine and so on.
- The properties of hydrogen, fluorine and chlorine are similar.
- Every eighth element of a certain element have similar chemical properties in the table of seven groups.
Question 5.
Electronic configuration of a molecule ‘X’ is 1s2 2s2 2p1. It should form XF molecule. But it forms . XF3. HOW can it possible ? What the ‘X’ may be ?
Answer:
1. ‘X’ is nothing Boran.
2. It has to form one covalent bond with fluorine because of one un paired electron.
3. But it undergoes excitation and forms 3 unpaired electrons and the electronic configuration
of excited boran is 1s2 2s1 2p12py1.
- Each carbon is only joining to two other atoms rather than four or three.
- Here the carbon atoms hybridise their outer orbitals before forming bonds, this time they only hybridizes two of the orbitals.
- They use the ‘s’ orbital (2s) and one of the 2p orbitals uncharged because they are made by an s-orbital and a p-orbital reorganizing themselves.
- The new hybrid orbitals formed are called sp-hybrid orbitals.
Question 6.
What is sp hybridisation ? Explain.
Answer:
- Each carbon is onlyjoining to two other atoms rather than four or three.
- Here the carbon atoms hybridise their outer orbitals before forming bonds, this time they only hybridizes two of the orbitals.
- They use the ‘s’ orbital (2s) and one of the 2p orbitals uncharged because they are made by an s-orbital and a p-orbital reorganizing themselves.
- The new hybrid orbitals formed are called sp-hybrid orbitals.
Section – III (3 × 5 = 15 Marks)
Instructions :
- 3 Essay answer questions are there in section.
- Answer ALL the questions. Each question carries 5 marks.
- Internal choice is given in this section.
- Write answer to each question in 8 to 10 sentences.
Question 7.
Write the required material and experimental procedure for the experiment, “Hydrochloric acid reacts with ‘Zn’ pieces and liberates H2O.
(OR)
List out the materials required to test whether the solutions of given acids and bases contain ions or not. Explain the procedure of the experiment.
Answer:
Aim: To show the reaction of acids with metals.
Required Materials :
1. Test tube,
2. Delivery tube,
3. Glass trough,
4. Candle,
5. Soap water,
6. Dil. HCl,
7. Zinc granules.
Procedure:
- Set the apparatus as shown in figure.
- Take about 10 ml of dilute HCl in a test tube and add a few zinc granules to it.
- We will observe the formation of gas bubbles on the surface of zinc granules.
- Pass the gas being evolved through the soap water.
- Gas filled bubbles are formed in the soap solution which rises into the air.
- Bring a burning candle near the gas filled bubbles.
- The gas present in a soap bubble burns with a ‘pop’ sound.
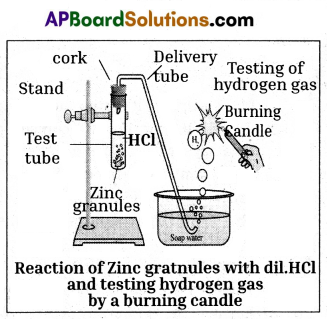
Result :
- Only ‘hydrogen’ gas burns making a ‘pop’ sound.
- So we will notice that gas evolved is H2.
Chemical reaction:
Acid + Metal → Salt + Hydrogen
2HCl(aq) + Zn(s) → ZnCl2(aq) + H2(g)
Additional Experiment:
- Repeat the above experiment with H2SO4 and HNO3.
- We will get the same observation of the HCl experiment.
Conclusion : From the above activities we can conclude that when acid reacts with metal, H2 gas is evolved.
(OR)
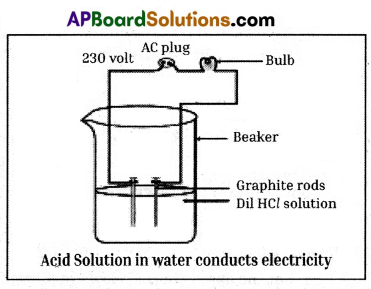
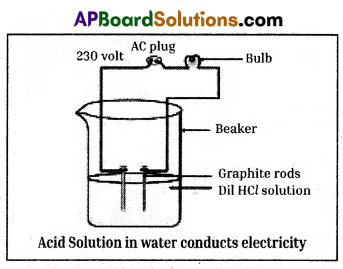
Required materials: 100 ml beaker, rubber cork, graphite rods, two different coloured wires, bulb,
glucose, alcohol, hydrochloric and sulphuric acids.
Procedure:
- Prepare glucose, alcohol, hydrochloric and sulphuric acid.solution.
- Fix two iron graphite rods on a rubber cork and place the cork in a 100 ml beaker.
- Connect two different coloured electrical wires to graphite rods separately as shown in figure.
- Connect free ends of the wire to 230 volts AC plug.
- Complete the circuit as shown in the figure by connecting a bulb to one of the wires.
- Now pour some dilute HCl in the beaker and switch on the current.
Observation : The bulb starts glowing.
Repeatation : Repeat activity with dilute sulphuric acid, glucose and alcohol solutions separately.
Observation:
- We will notice that the bulb glows only in acid solutions.
- But the bulb not glows in glucose and alcohol solutions.
Result :
- Glowing of bulb indicates that there is flow of electric current through the solution.
- Acid solutions have ions and the movement of these ions in solution helps for flow of electric current through the solution.
Conclusion :
- The positive ion (cation) present in HCl solution is H+.
- This suggests that acids produced hydrogen ions H+ in solution, which are responsible for their acidic properties.
- In glucose and alcohol solutions the bulb did not glow indicating the absence of H+ ions in these solutions.
- The acidity of acids is attributed to the H+ ions produced by them in solutions.
![]()
Question 8.
Radii of biconvex lens are equal. Let us keep an object at one of the centres of curvature. Refractive index of lens is ‘n’. Assume lens in the air. Let us take R as the radius of the curvature.
a) How much is the focal length of the lens?
b) What is the image distance ?
c) Discuss the nature of the image.
(OR)
Make the s, p and d-orbital models.
Answer:
Radii of curvatures (R) of biconvex lens are equal, so, R1 = R2 = R
a) According to lens formula
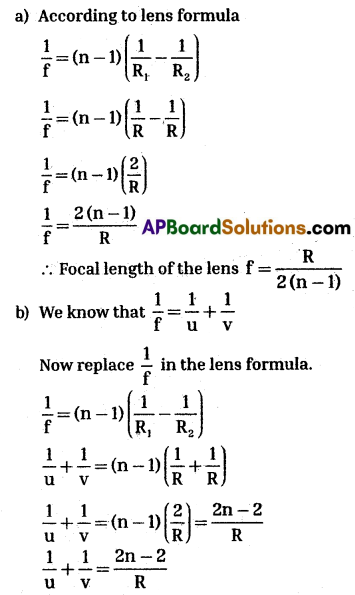
Object is placed at centre of curvature.
So, object distance u = R
Let the image distance v = v
From above equation
\(\frac{1}{v}=\frac{2 n-2}{R}-\frac{1}{u}=\frac{2(n-1)}{R}-\frac{1}{R}\)
\(\frac{1}{v}=\frac{2 n-2-1}{R}-\frac{2 n-3}{R}\)
∴ Image distance = v = \(\frac{R}{2 n-3}\)
c) The nature of the image is inverted and v < u.
(OR)
Materials required: lo Iron spokes, 9 i’ound shaped wooden pieces, dumb-bell shaped beeds having hole along its length.
Procedure :
- Cut the each iron spoke into three pieces in which one of them is longer than the other two.
- Make a hole at the middle of the wooden piece and insert the long spoke into that hole.
- Fix the other two-spokes to the longer spoke horizontally such that the three spokes are perpendicular to each other.
- Now the three spokes represents x, y and z-axis.
- Make nine of this types of models, one for s-orbital, three for p-orbitals and five for d-orbitals,
- Fix the dumb-bell shaped beeds to the iron spokes as shown in the fig (a) – to make three (Px Py, Pz) P – orbitals.
- Fix the dumb-bell shaped beeds to the iron spokes as shown in the fig (b) – to make five d-orbitals. (dxy dyz, dzx, dx2 – dy2 and dz2)
Question 9.
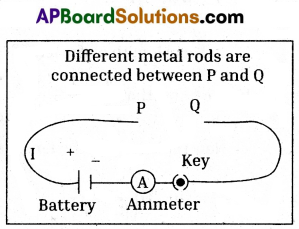
How do you verify that resistance of a conductor of uniform cross-section area is proportional to the length of the conductor at constant temperature.
(OR)
Collect information of experiments done by Faraday. (Or) What is the information do you collect of experiments done by Faraday ?
Answer:
- To verify the relation between resistance and length of the conductor. Collect some wires or spokes of different lengths with same cross-section area of the same metal.
- Construct a circuit with Battery, Ammeter, Switch (key) and connecting wires, keeping some space at the both ends.
- Connect the selected wires or spokes at the ends to complete the circuit.
- Connect the wires or spokes individually and record the current using ammeter.
- If the current flowing in the circuit decreases with an increase in the length of the wire or spokes (Resistance increases), we can say that the resistance of the conductor is proportional to the length of the conductor.
(OR)
Experiment – 1
- Connect the terminals of a coil to a sensitive galvanometer.
- Normally, we would not expect any deflection of needle in the galvanometer because there is no EMF in the circuit.
- Now, if we push a bar magnet towards the coil, with its north pole facing the coil, the needle in the galvanometer deflects, showing that a current has been set up in the coil, the galvanometer does not deflect if the magnet is at rest.
- If the magnet is moved away from the coil, the needle in the galvanometer again deflects, but in the opposite direction, which means that a current is set up in the coil in the opposite direction.
- If we use the end of south pole of a magnet instead of north pole, the results i.e., the deflections in galvanometer are exactly opposite to the previous one.
- This activity proves that the change in magnetic flux linked with a closed coil, produces current.
- From this Faraday’s law of induction can be 1 stated as “whenever there is a continuous change of magnetic flux linked with a closed coil, a current is generated in the coil.” This induced EMF is equal to the rate of change of magnetic flux passing through it.
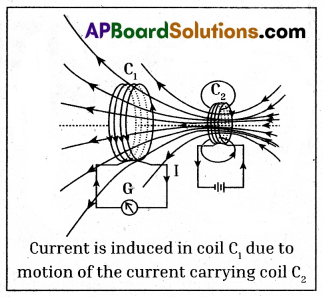
Experiment – 2
- Prepare a coil of copper wire C1 and connect the two ends of the coil to a galvanometer.
- Prepare another coil of copper wire similar to C2 and connect the two ends of the coil to a battery via switch.
- Now arrange the two coils C1 and C2 nearby as shown in the figure.
- Now switch on the coil C2. We observe a deflection in the galvanometer connected to the coil C1.
- The steady current in C2 produces steady magnetic field. As coil C2 is moved towards the coil C1, the galvanometer shows a deflection.
- This indicates that electric current is induced in coil C1.
- When C2 is moved away, the galvanometer shows a deflection again, but this time in the opposite direction.
- The deflection lasts as long as coil C2 is in motion.
- When C2 is fixed and C1 is moved, the same effects are observed.
- This shows the induced EMF due to relative motion between two coils.
![]()
Part – B (10 × 1 = 10 Marks)
Instructions :
- Answer ALL the questions.
- Each question carries 1 mark.
- In this section there are 4 options (A / B / C / D) to each question. Choose the appropriate answer and
write the answer in the brackets given against the question. Part – B must be attached to the answer booklet of Part – A.
Question 1.
Starting from a long distance, a flame Is moved towards a convex mirror. Then the image
A) Decreases in size and moves towards pole
B) Increase in size and moves towards pole
C) Decrease in size and moves away from pole
D) Increase in size and moves away from pole
Answer:
B) Increase in size and moves towards pole
Question 2.
In exothermic reactions heat energy is
A) absorbed
B) created
C) released
D) none of these
Answer:
C) released
Question 3.
The acid which enters the body by the sting of bee is
A) Acetic acid
B) Methanoic acid
C) Sulphuric acid
D) Fatty acid
Answer:
B) Methanoic acid
Question 4.
An aqueous solution of the salt is acidic which of the following acids and bases react to give this salt?
A) Strong acid and strong base
B) Strong acid and weak base
C) Weak acid and strong base
D) Weak acid and weak base
Answer:
B) Strong acid and weak base
Question 5.
Select a pair of basic salts among the following.
A) Sodium chloride and Sodium acetate
B) Sodium acetate and Sodium bicarbonate
C) Sodium carbonate and Sodium sulphate
D) Sodium nitrate and Sodium oxalate
Answer:
B) Sodium acetate and Sodium bicarbonate
Question 6.
Which of the following lens act as converging lenses ?
A) Bi-convex lens
B) Plano-convex lens
C) Concave convex
D) All the above
Answer:
D) All the above
![]()
Question 7.
Which one of the following is normally used for the preparation of lenses?
A) Water
B) Glass
C) Plastic
D) All of these
Answer:
B) Glass
Question 8.
A person with ……… can see distances objects usually but cannot see objects at near distances.
A) myopia
B) hypermetropia
C) presbyopia
D) none of these
Answer:
B) hypermetropia
Question 9.
1 pm = …………..
A) 10-11
B) 10-12
C) 10-13
D) 10-18
Answer:
B) 10-12
Question 10.
Food cans are coated with tin and not with zinc because
A) zinc is less reactive than tin
B) zinc is costlier than tin
B) zinc is costlier than tin
D) zinc is more reactive than tin
Answer:
D) zinc is more reactive than tin