Timed practice with TS 10th Class Physical Science Model Papers Set 2 is crucial for improving speed and efficiency during exams.
TS 10th Class Physical Science Model Paper Set 2 with Solutions
Time: 1 Hour 30 minutes
Maximum Marks: 40
General Instructions:
- Read the question paper and understand every question thoroughly and write answers in given 1.30 hrs. time.
- 3 very short answer questions are there in section – I. Each question carries 2 marks. Answer all the questions. Write answer to each question in 3 to 4 sentences.
- 3 short answer questions are there in section – II. Each question carries 3 marks. Answer all the questions. Write answer to each question in 5 to 6 sentences.
- 3 essay type answer questions are there in section – III. Each question carries 5 marks. Answer all the questions. Write answer to each question in 8 to 10 sentences. Internal choice is given in this section.
Part – A (30 Marks)
Section – I (3 × 2 = 6 Marks)
Instructions :
- 3 Very short answer questions are there in section – I
- Answer ALL the questions. Each question carries 2 marks.
- Write answer to each question in 3 to 4 sentences.
Question 1.
Why do we prefer a convex mirror as a rearview mirror in vehicles?
Answer:
We prefer a convex mirror as a rear-view mirror in vehicles because of the following reasons.
- A convex mirror always forms an erect, virtual and diminished image of an object placed at anywhere in front of it.
- A convex mirror has a wider field of view than a plane mirror of the same size.
- Thus convex mirrors enable the driver to view much larger traffic behind him than would be possible with a plane mirror.
Question 2.
A rainbow viewed from an airplane may form a complete circle. Where will the shadow of the airplane appear ? Explain.
Answer:
- A rainbow viewed from an airplane form a complete circle because the earth does not come along the way of the airplane and rainbow.
- A rainbow is a three dimensional cone of dispersed light it appear as a complete circle.
- The shadow of the airplane appear with in the circle of the rainbow.
![]()
Question 3.
Explain the reaction of various metals in activity with cold water.
Answer:
- From potassium to magnesium displace, hydrogen from cold water with decreasing reactivity. Potassium react with cold water violently but reaction of magnesium is very slow. The reactivity order is given below.
Mg < Ca < Na < K - From Aluminium to gold hydrogen from cold water is not displaced.
Section – II (3 × 3 = 9 Marks)
Instructions :
- 3 Short answer questions are there in section – II.
- Answer ALL the questions. Each question carries 3 marks.
- Write answer to each question in 5 to 6 sentences.
Question 4.
55Cs has less ionization energy than 3Li. How can you explain it ?
Answer:
- It is because of screening effect.
- Atomic number of Cs is 55, means it has more electrons and more inner shells.
- Between nucleus and valence electron, these shells act as screens and decrease nuclear attraction.
- So 55Cs has less ionization energy than 3Li.
Question 5.
A chemical compound has the formula AB3. Identify the molecule.
a) Without any lone pair’s.
b) With one lone pair.
c) Mention the shape of any one of the above molecules.
Answer:
a) BF3, BCl3, AlCl3 etc.
b) NH3, PH3, PCl3 etc.
c) NH3, PH3, PCl3 – Pyramidal Shape
BF3 – Traingle Planar Shape
Question 6.
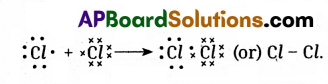
Draw the structure of Ethane and electron dot structure of Chlorine.
Answer:
Ethane:
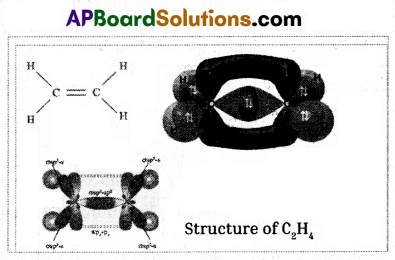
Chlorine:
![]()
Section – III (3 × 5 = 15 Marks)
Instructions :
- 3 Essay answer questions are there in section.
- Answer ALL the questions. Each question carries 5 marks.
- Internal choice is given in this section.
- Write answer to each question in 8 to 10 sentences.
Question 7.
How to make a chemical equation more informative ?
(OR)
Distinguish between acids and bases.
Answer:
Chemical equations can be made more informative by expressing the following characteristics of the reactants and products.
i) Physical state
ii) Heat exchange (exothermic or endothermic change
iii) Gas evolved (if any)
iv) Precipitate formed (if any)
i) Expressing the physical state : The physical state of the substances may be mentioned along with their chemical formula. The different states i.e., gaseous, liquid and solid states are represented by the notations (g), (l) and (s) respectively. If the substance is present as a solution in water, it is represented as (aq).
E.g.: Fe2O3(s) + 2 Al(s) → 2 Fe(s) + Al2O3(s)
ii) Heat exchange : Heat is liberated in exothermic reactions and heat is absorbed in endothermic reactions.
1) C(s) + O2(g) → CO2(g) + Q (exothermic reaction)
2) N2(g) + O2(g) → 2NO(g) – Q (endothermic reaction)
(Or) N2(g) + O2(g) ![]() 2NO(g)
2NO(g)
iii) Gas evolved : Ifa gas is evolved ma reaction, it is denoted by an upward arrow t or (g).
E.g.: Zn(s) + H2SO4(aq) → ZnSO4(aq) + H(2g)↑
iv) Precipitate formed: If a precipitate is formed in the reaction, it is denoted by a downward arrow ↓.
E.g.: AgNO3(aq) + NaCl(aq) AgCl(s)↓ + NaNO3(aq)
(OR)
| Acids | Bases |
| 1. An acid is a substance whichgives H30+ ions in Water solution | 1. A base is a solution which contains OH group in water solution gives hydroxyl ions OH– |
| 2. Acids are sour in taste | 2. Bases are bitter in taste. |
| 3. Aciaturns blue litmus to red. | 3. Bases turn red litmus in taste |
| 4. The orange colour of methyl orange indicator changes to red in acid medium | 4. The orange colour of methyl orange indicator changes to yellow in bases medium. |
| 5. Acid are formed when non-metal oxides are dissolved in water | 5. Bases are formed when metal oxides are dissolved in water |
| 6. pH value of acid is less than 7 | 6. pH value of base is geater than 7 |
Question 8.
Write characteristics of image formed due to convex lens at various distances.
(OR)
Distinguish between emission and absorption spectrum.
Answer:
| Position of the object | Position of the image | Relative size of the image | Nature of the image |
| 1) At infinity | Focal point | Highly diminished | Real and inverted |
| 2) Beyond centre of curvature (C2) | Between F1 and C1 | Point size diminished | Real and inverted |
| 3) At the centre pf curvature (C2) | At C1 | Same size | Real, inverted |
| 4) Between centre of cur-vature focal point (C2 and F2) | Beyond centre of curvature | Magnified | Real, inverted |
| 5) At focal point (F2) | At infinity | Highly magnified | Real and inverted |
| 6) Between focal point and pole F2 and P | On the same side of the lens of the object | Magnified | Virtual and erect |
(OR)
| Emission spectrum | Absorption spectrum |
| 1) The spectrum produced by emitted radiation is called emission spectrum. | 1) The spectrum produced by absorption of radiation is called absorption spectrum. |
| 2) The emission spectrum contains bright lines on the dark background | 2) The absorption spectrum contains dark lines on the bright background. |
| 3) The emission spectrum corresponds the radiation emitted when an excited electron returns back to the ground state. | 3) The absorption spfectrum coresponds the radiation absorbed in exciting an electron from lower to the higher energy levels. |
Question 9.
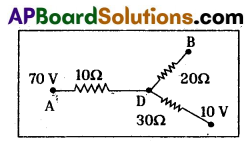
Observe the picture. The potential values at A, B, C are 70V, 0V, 10V
a) What is the potential at D ?
b) Find the ratio of the flow of current in AD, DB, DC.
(OR)
List out the apparatus and experimental procedure for the experiment to observe a current carrying wire experiences a magnetic force when it is kept in uniform magnetic field.
i) pushed into the coil ?
ii) withdrawn from inside the coil?
iii) held stationary inside the coil ?
Answer:
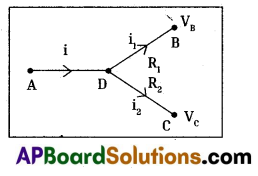
a) By following Ohm’s law P.D is (V) = iR
In the given circuit, we are applying junction laws.
‘D’ works as junction so, i = i1 + i2
Let P.D at D is V0,
We know that, i = \(\frac{\mathrm{V}}{\mathrm{R}}\)
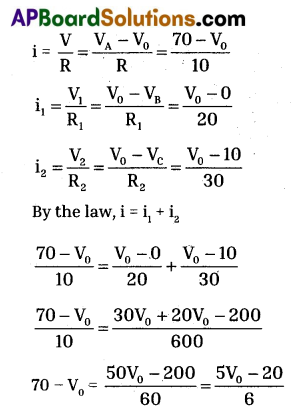
(70 – V0)6 = 5V0 – 20
420 – 6V0 = 5V0 – 20
6V0 + 5V0 = 440 ⇒ 11V0 = 440
V0 = \(\frac{440}{11}\) = 40
∴ Potential at D is = V0 = 40V
b) Flow of current in AD is
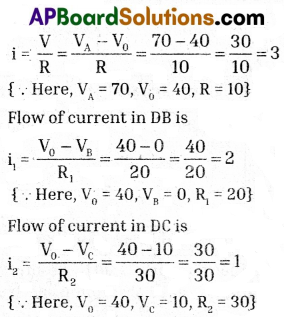
The ratio of the flow of current in AD, DB and DC is 3 : 2 : 1.
(OR)
- A copper ware is passed through splits of wooden sticks.
- Connect the wire to 3 volts battery.
- Close the switch of the battery and pass the current.
- Bring the horse-shoe magnet near the wire.
- Then a force is experienced on the wire.
- Reverse the polarities of the magnet, then the direction of the force is also reversed.
- The right hand rule helps the direction of flow of current and the direction of current.
- Due to the uniform magnetic field of horse-shoe magnet, current carrying wire moves in opposite side to the magnet.
- Here the wire bends downwards due to the force applied by the magnet.

![]()
Part – B (10 × 1 = 10 Marks)
Instructions :
i) Answer ALL the questions.
ii) Each question carries 1 mark.
iii) In this section there are 4 options (A / B / C / D) to each question. Choose the appropriate answer and write the answer in the brackets given against the question. Part – B must be attached to the answer booklet of Part – A.
Question 1.
When light gets reflected from a surface, If the angle of Incidence is 30°, then what about the angle of reflection?
A) 30°
B) 45°
C) 60°
D) 150°
Answer:
A) 30°
Question 2.
The chemical reaction in which energy is absorbed to form a new compound is called ………..
A) exothermic reaction
B) endothermic reaction
C) thermal reaction
D) photochemical reaction
Answer:
B) endothermic reaction
Question 3.
Which of the following is used for making toys?
A) Gypsum
B) Calcium carbonate
C) Plaster of Paris
D) Bleaching powder
Answer:
B) Calcium carbonate
Question 4.
Which of the following gas is liberated, when sodium hydroxide reacts with zinc ?
A) H2
B) N2
C) O2
D) None of these
Answer:
A) H2
Question 5.
Bases which are soluble in water are called
A) Acidic
B) Basic
C) Alkali
D) Neutral
Answer:
C) Alkali
Question 6.
Two plane convex lenses of focal lengths 10 cm and 20 cm are placed in contact with each other. The effective focal length of the combination is
A) 10 cm
B) 20 cm
C) 200 cm
D) 6.67 cm
Answer:
D) 6.67 cm
![]()
Question 7.
What is value of image distance (v), if focal length (f) is 20 cm ahd object distance (u) is 30 cm is placed on the principal axis of convex lens ?
A) 60 cm
B) 80 cm
C) 40 cm
D) 20 cm
Answer:
A) 60 cm
Question 8.
The power of accommodation for the normal eye is
A) 400 D
B) 4D
C) 40 D
D) 44 D
Answer:
B) 4D
Question 9.
Cl belongs to ………………. family.
A) noble gas
B) carbon, family
C) halogen
D) boron
Answer:
C) halogen
Question 10.
The most abundant metal in the earth’s crust is
A) Silver
B) Aluminium
C) Zinc
D) Iron
Answer:
B) Aluminium