Students get through AP Inter 1st Year Chemistry Important Questions 2nd Lesson Classification of Elements and Periodicity in Properties which are most likely to be asked in the exam.
AP Inter 1st Year Chemistry Important Questions 2nd Lesson Classification of Elements and Periodicity in Properties
Very Short Answer Questions
Question 1.
What is the difference in the approach between the Mendeleev’s periodic law and the modern periodic law?
Answer:
According the Mendeleev, the physical and chemical properties of elements are periodic functions of their atomic weights.
According to modern periodic law, the physical and chemical properties of elements are periodic functions of their atomic numbers.
Question 2.
In terms of period and group, where would you locate the element with Z = 114?
Answer:
Electronic configuration of the element with Z = 114 is [Rn] 5f146d10 7s² 7p²
Period number = Valence Shell number = 7
Group number = No.of valence electrons = 4
So the element belongs to 7th period and IVA group, in the p-block
Question 3.
Write the atomic number of the element, present in the third period and 17th group of the periodic table.
Answer:
The valency shell configuration of the element in 3rd period & 17th group(VIIA) is 3s²3p5.
Its Electronic configuration is [Ne]3s²3p5
∴ It’s atomic number =10 + 2 + 5 = 17(Cl).
Question 4.
Which element do you think would have been name by (a) Lawrence Berkeley Laboratory (b) Seaborg’s group?
Answer:
(a) Lawrencium Lr (element 103)
(b) Seaborgium Sg (element 106)
Question 5.
Why do elements in the same group have similar physical and chemical properties?
Answer:
In a group, all the elements have same outer electronic configuration. Hence they have similar physical and chemical properties.
![]()
Question 6.
What are representative elements? Give their valence shell configuration.
Answer:
Elements of s and p block, excluding ‘0’ group are called ‘representative elements’. Their valence shell configuration is ns1-2np1-5.
Question 7.
Justify the position of f-block elements in the periodic table.
Answer:
The two series of f-block elements Lanthanides and Actinides are grouped separately and placed at the bottom of the periodic table, though they belong to the sixth and seventh periods of third group (III B).
This placement is done on the basis of their similar properties. The properties are so similar that elements from Ce to Lu can be considered as equivalent to one element. In case, if these elements are assigned the usual positions, the symmetry of the whole arrangement of the periodic table would be disrupted.
Question 8.
An element ‘X’ has atomic number 34. Give its position in the periodic table.
Answer:
Electronic configuration of X : [Ar] 3d104s²4p4.
Valency shell configuration is 4s²4p4.
Period number = Valence Shell number = 4
Group number = No.of valence electrons = 6
∴ X belongs to 4th period and VIA group.
Question 9.
What factors impart characteristic properties to the transition elements?
Answer:
Characteristic properties of transition elements:
a) small atomic size
b) high nuclear charge
c) unpaired electrons in ‘d’ orbitals.
Question 10.
Give the outer shells configuration of d-block and f- block elements.
Answer:
The general electronic configuration of d-block elements is (n – 1) d1-10 ns1-2.
The general outer electronic configuration of f-block elements is (n – 2)f1-14 (n – 1)d0-1ns²
![]()
Question 11.
State and give one example for Dobereiner’s law of triads and Newland’s law of octaves.
Answer:
a) Dobereiner’s law of triads :
When a group of three elements of similar properties are arranged in the increasing order of their atomic weights, the atomic weight and properties of middle element is arithmetical mean of the other two
![]()
b) Newland’s law of octaves :
When elements are arranged in the increasing order of their atomic weights, every succeeding 8th element is a repetition of the first one, just like the eighth note in an octave of musical scale.
Question 12.
Name the anomalous pairs of elements in the Mendeleev’s periodic table.
Answer:
Anomalous Pairs:
Ex: 1) Ar (40); K (39)
2) Te (127.6); I (126.9)
3) Co (58.93); Ni (58.69)
Here, the second element in each pair has less atomic weight than the first.
Question 13.
How does atomic radius vary in a period and in a group? How do you explain the variation?
Answer:
Atomic radius :
It is the average distance between the centre of nucleus and the outermost shell of the atom.
In a period :
Atomic radius decreases from left to right, across a period.
Reason :
In a period, the differentiating electron enters into the same shell. Hence the effective nuclear charge increases.
In a group :
Atomic radius increases from top to bottom in a group.
Reason :
In a group, the differentiating electron enters into a new shell. Hence the atomic size increases.
Question 14.
Among N-3, O-2, F– Na+, Mg+2 and Al+3.
(a) What is common in them?
(b) Arrange them in the increasing ionic radii.
Answer:
Given ions: N-3, O-2, F– Na+, Mg+2, Al+3.
(a) All the given ions have the same number of electrons (10 electrons each).
These are called iso-electronic species.
(b) The increasing order of ionic radius:
Al+3 < Mg+2 < Na+ < F– < O-2 < N-3
Reason :
In case of iso-electronic species the ionic radius decreases with the increase of the effective nuclear charge.
Question 15.
What is the significance of the term ‘isolated gaseous atom’ while defining the ionization enthalpy.
(Hint: Requirement for comparison)
Answer:
It is useful to compare the ionisation enthalpies of atoms of different elements.
Reason :
Isolated gaseous atom means a single gaseous atom free from other atoms. No energy should be utilised to separate it further from other atoms
![]()
Question 16.
Energy of an electron in the ground state of the hydrogen atom is -2.18 × 10-18J. Calculate the ionization enthalpy of atomic hydrogen in terms of J mol-1.
Answer:
Energy of electron = -[Ionisation energy]
I.E interms of J mol-1
= 2.18 × 10-18J × 6.023 × 1023 = 3.13 × 105Jmol-1
Question 17.
Ionization enthalpy (IEJ) of O is less than that of N. Explain.
Answer:
E.C ofN is 1s²2s²2p³.
E.C of O is 1s² 2s² 2p4
Thus N has stable electronic configuration due to half-filled orbitals.
Relatively, O has less stable electronic configuration when compared to ‘N’
So IE1 of O is less that of N.
Question 18.
Which in each pair of elements has a more negative electron gain enthalpy?
(a) O or F (b) F or Cl [TS 22]
Answer:
a) Fluorine has more negative electron gain enthalpy than that of Oxygen,
b) Chlorine has more negative electron gain enthalpy than that of Fluorine.
Question 19.
What are the major differences between metals and non-metals?
Answer:
| Metals | Non-Metals |
| 1) Metals are usually solids at room temperature (except Hg). | 1) Non metals are usually solids (or) gases at room temperature. |
| 2) Metals are good conductors of heat and electricity. | 2) Non- Metals are poor conductors of heat and electricity. |
| 3) Metals have high M.P and B.P | 3) Non- Metals have low M.P and B.P |
| 4) Generally these are electropositive. | 4) Generally these are electronegative. |
| 5) These forms more ionic compounds. | 5) These forms more covalent compounds. |
![]()
Question 20.
Use the periodic table to identify elements
(a) with 5 electrons in the outer subshell
(b) Would tend to lose two electrons
(c) Would tend to gain two electrons
Answer:
Group number = No.of valence electrons
a) If the outer subshell contains 5 electrons then it should belong to VA group.
b) If an element tend to lose two electrons then its atom contains two electrons in the outer orbit. So it belongs to 2nd group.
c) Let an element tend to gain two electrons to get octet to acquire stability. Since the element tends to gain two electrons its should have 6 electrons in its outer orbit.
So the element belongs to VIA group.
Question 21.
Give the outer electronic configuration of s, p, d and f-block elements.
Answer:
| Block | Outer E.C |
| a) s-block | ns1-2 |
| b) p-block | ns2np1-6 |
| c) d-block | ns1-2(n-1)d1-10 |
| d) f-block | ns2(n-1)d0 or 1 (n-2)f1-14 |
Question 22.
Write the increasing order of the metallie character among the elements B, Al, Mg and K.
Answer:
Increasing order of metallic character:
![]()
Question 23.
Write the correct increasing, order of lion-metallic character for B, C, N, F, Si.
Answer:
Increasing order of non-metallic character:
![]()
Question 24.
Write the correct increasing order of chemical reactivity in terms of oxidizing property of N, O, F and Cl.
Answer:
Increasing order of chemical reactivity :
F > O > Cl > N.
Question 25.
What is electro negativity? How is this useful in understanding the nature of elements?
Answer:
The tendency of an atom to attract the shared pair of electrons towards itself in a diatomic molecule is called electronegativity.
Electronegativity is inversely proportional to the metallic nature of elements. Hence the nature and reactivity of an element can be estimated with the help of electronegativity.
Question 26.
What is Screening effect? How it is related to IE?
Answer:
The electrons present in the inner shells act as screens between the nucleus and the valence shell electrons. In other words, these electrons partially neutralise the force of attraction of the nucleus, over the valence electrons. This is called, ‘shielding or screening effect’ of inner shells.
When the screening effect increases, the IE value decreases.
![]()
Question 27.
How are electronegativity and metallic & non-metallic characters related?
Answer:
Electronegativity ∝ Non-metallic nature
![]()
As the electronegativity increases across a period from left to right, the metallic character decreases and non-metallic character increases.
In a group electronegativity decreases from top to bottom. So the metallic character increases down and the non-metallic character decreases.
Question 28.
What is the valency possible to Arsenic with respect to oxygen and hydrogen?
Answer:
The valency of Arsenic with respect to Oxygen is ‘3’, ‘5’
Ex: AS2O3, AS2O5.
The valency of Arsenic with respect to Hydrogen is ‘3’
Ex: AsH3.
Question 29.
What is an amphoteric oxide? Give the formula of an amphoteric oxide formed by an element of group-13,
Answer:
The oxide having both basic and acidic nature is called amphoteric oxide.
The amphoteric oxide with ‘Al’ of group 13 is Al2O3.
Question 30.
Name the most electronegative element. Is it also having the highest electron gain enthalpy? Why or Why not?
Answer:
The most electronegative element is Fluorine. It does not have the highest electron gain enthalpy. It is due to low atomic size and strong inter electronic repulsions.
Question 31.
What is a diagonal relationship? Write an example. [TS 22]
Answer:
Diagonal relationship :
The elements of 2nd period have certain similarities with the elements situated diagonally in the third period. This is called diagonal relationship.
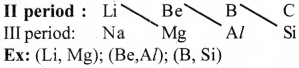
Question 32.
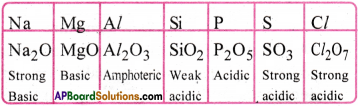
How does the nature of oxides change along third period from Na2O to Cl2O7?
Answer:
From Na2O to Cl2O7, basic character decreases and acidic character increases
Question 33.
Radii of iron atom and its ions follow Fe > Fe+2 > Fe+3 Explain?
Answer:
When the +ve charge increases on a metal atom, it looses electrons. Then its effective nuclear charge increases and hence size decreases.
Question 34.
IE2 > IE1 for a given element- why?
Answer:
After removal of one electron, the effective nuclear charge increases on the remaining electrons. As a result, attraction between nucleus and outer electron increases. So IE2 > IE1.
![]()
Question 35.
What is lanthanide contraction? What are its consequences?
Answer:
The steady decrease of atomic radii of Lanthanide series, due to poor shielding effect and peculiar shape of f-orbitals, is called Lanthanide contraction.
Consequences :
- The crystal structure and other properties of these elements become very close and similar. So it becomes difficult to separate them from a mixture.
- Inert pair effect is consequence of Lanthanide contraction.
Question 36.
What is the atomic number of the element, having maximum number of unpaired 2p electrons? To which group does it belong?
Answer:
The atomic number of the element having maximum no. of unpaired 2p electrons is ‘7’ (Nitrogen)
Its Electronic configuration is 1s²2s²2p³
The number of valence electrons = 2 + 3 = 5
∴ Nitrogen belongs to group-15.
Question 37.
Sodium is strongly metallic, while Chlorine is strongly non-metallic. Explain.
Answer:
Atomic number of Sodium is 11.
Its Electronic configuration is 1s²2s²2p63s¹ So it easily looses its valency electron for stability. So it exhibits more electro positive in nature. Hence Na is strongly metallic.
Atomic number of Chlorine is 17. Its Electronic configuration is 1s²2s²2p63s²3p5 So it gains electron for stability. So it exhibits more electronegative in nature.
Hence Cl is strongly non-metallic.
Question 38.
Why are zero group elements called noble gases or inert gases?
Answer:
Zero group elements have stable ns²np6 outer electronic configuration.
So these elements are chemically inert. Hence these are called inert gases.
These elements are less reactive like noble metals such as Gold and Platinum. Hence they are also called noble gases.
Question 39.
Select in each pair, the one having lower ionization energy and explain the reason.
(a) I and I–
(b) Br and K
(c) Li and Li+
(d) Ba and Sr
(e) O & S
(f) Be and B
(g) N and O
Answer:
(a) I–
Reason:
1 has larger size and less effective nuclear charge than I
(b) K
Reason :
In a period, from left to right, I.E generally increases.
(c) ‘Li’
Reason :
Effective nuclear charge of Li is less than Li+
(d) Ba
Reason :
In a group, from top to bottom, IE decreases.
(e) ‘S’
Reason :
In a group, from top to bottom, IE decreases.
(f) ’B’
Reason :
’Be1 has completely filled electronic configuration (1s²2s²)
(g) ’O’
Reason :
‘N’ is more stable due to half-filled electronic configuration (1s²2s²2p³)
![]()
Question 40.
IE1 of O < IE1 of N. But IE2 of O > IE2 of N. Explain.
Answer:
’N’ is more stable due to half-filled electronic configuration (1s²2s²2p³)
But Oxygen is less stable as it does not contain half-filled configuration.
So IE1 of O < IE1 of N.
After removal of an electron, Oxygen acquires stable [He]2s²2p³ half-filled configuration.
So IE2 of O > IE2 of N
Question 41.
Na+ has higher value of ionization energy than Ne, though both have same electronic configuration. Explain.
Answer:
In Na+, the number of protons = 11 and number of electrons =10.
In Ne, the number of protons = 10 and number of electrons =10.
Thus Na+ has more number of protons. Thus, effective, nuclear charge on the valence electrons increases and hence its size decreases.
∴ Na+ has high Ionization energy than Ne.
Question 42.
Which in each pair of elements has a more electronegative gain enthalpy? Explain (a) N or O (b) F or Cl
Answer:
(a) Oxygen
Reason :
‘N’ has stable half-filled electron configuration and hence it is reluctant to gain electrons.
(b) Chlorine
Reason :
Electron affinity of Chlorine is greater than Fluorine. It is due to exceptionally smaller size and strong inter electronic repulsions in 2p subshell of Fluorine.
Question 43.
Electron affinity of Chlorine is more than that of Fluorine. Explain.
Answer:
Electron affinity of Chlorine is greater than Fluorine. It is due to exceptionally smaller size and strong inter electronic repulsions in 2p sub shell of Fluorine.
Question 44.
Which in each has higher electron affinity?
a) F or Cl– b) O or O–
c) Na+ or F
d) F or F–
Answer:
a) F b) O c) F d) F
Question 45.
Arrange the following in order of increasing ionic radius:
a) Cl–, P-3, S-2, F–
b) Al+3, Mg+2, Na+, O-2, F–
c) Na+ , Mg+2, K+.
Answer:
Increasing order of ionic radii:
a) F– < Cl– < S-2 < P-3
b) Al+3 < Mg+2 < Na+ < F– < O-2
c) Mg+2 < Na+ < K+
![]()
Question 46.
Mg+2 is smaller than O-2 in size, though both have same electronic configuration Explain.
Answer:
In Mg+2, the number of protons = 12 and number of electrons =10.
In O-2, the number of protons = 8 and number of electrons = 10.
Thus Mg+2 has more number of protons. Thus, effective nuclear charge on the valence electrons increases and hence its size decreases.
Thus Mg+2 has small size than O-2.
Question 47.
Among the elements B, Al, C and Si.
a) Which has the highest first ionization enthalpy
b) Which has the most negative electron gain enthalpy?
c) Which has the largest atomic radius
d) Which has the most metallic character?
Answer:
(a) C
(b) C
(c) Al
(d) Al.
Question 48.
Consider the elements N, P, O and S and arrange them in order of
a) Increasing first ionization enthalpy
b) Increasing negative electron gain enthalpy
c) Increasing non-metallic character.
Answer:
Increasing order of I.P :
(a) S < P < O < N
Increasing order of EA:
(b) N < P < O < S
Increasing order of non – metallic character:
(c) P < S < N < O.
Question 49.
Arrange in given order:
a) Increasing EA : O, S and Se
b) Increasing IE1 : Na, K and Rb
c) Increasing radius: I–, I+ and I
d) Increasing electronegativity : F, Cl, Br, I
e) Increasing EA : F, Cl, Br, I
t) Increasing radius: Fe, Fe+2, Fe+3.
Answer:
(a) Increasing order of EA : 0<Se<S
(b) Increasing order of IE1 : Rb < K < Na
(c) Increasing order of radius : I+ < I < I–.
(d) Increasing order of EN : I < Br < Cl < F
(e) Increasing order of EA : I < Br < F < Cl
(f) Increasing order of radius : Fe+3 < Fe+2 < Fe.
Question 50.
a) Name the element with highest ionization enthalpy.
b) Name the family with highest value of ionization enthalpy.
c) Which element possesses highest electron affinity?
d) Name unknown elements at the time of Mendeleev.
e) Name any two typical elements.
Answer:
a) ‘Helium’has the highest I.P.
b) ‘Zero group’ is the family with highest I.P
c) ‘Chlorine’ has the highest electron affinity.
d) Unknown elements at Mendeleev’s time:
i) Scandium (Eka -Boron)
ii) Germanium (Eka- Silicon)
iii) Gallium (Eka-Aluminium)
e) Typical elements(Elements of third period)
Ex: Sodium, Magnesium.
![]()
Question 51.
a) Name any two bridge elements
b) Name two pairs showing diagonal relationship
c) Name two transition elements
d) Name two rare earths
e) Name two transuranic elements.
Answer:
(a) Bridge elements (Elements of 2nd period)
Ex: Li, Be
(b) Diagonally related pairs:
Ex: (i) Li-Mg (ii) Be -Al.
(c) Transition Elements:
Ex: Scandium(Sc),Titanium(Ti).
(d) Rare Elements (Lanthanides):
Ex: Cerium (Ce), Promethium (Pm)
(e) Transuranic Elements:
Ex: Neptunium (Np), Californium (Cf).
Short Answer Questions
Question 1.
On the basis of quantum numbers, justify that the 6th period of the periodic table should have 32 elements.
Answer:
From the Aufbau principle, using the (n + l) rule, the increasing order of energies of various orbitals in the 6th period: 6s < 4f < 5d < 6p Thus, the subshells of elements in 6th period are 6s, 4f, 5d, 6p
6s can accommodate 2 electrons.
4f can accommodate 2 × 7 = 14 electrons
5d can accommodate 2 × 5 = 10 electrons
6p can accommodate 2 × 3 = 6 electrons
The total number of electrons that can be accommodated in the 6th period is 2 + 14 + 10 + 6 = 32
∴ The 6th period of the periodic table contains 32 elements.
Question 2.
How did Mosley’s work on atomic numbers show that atomic number is a fundamental property better than atomic weight?
Answer:
Mosley conducted various experiments by bombarding various elements with cathode rays in discharge tube.
They resulted X-rays with characteristic frequencies. Hence, he derived the equation
√v = a(Z – b), where v is the frequency of lines in the spectrum and Z is the atomic number of the corresponding element, a, b are constants for a selected series of lines in the X-ray spectrum.
The graph plotted between √v and atomic number Z resulted a straight line.
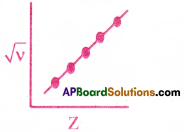
But when a graph is plotted between √v and atomic weight, he did not get a straight line. Hence Mosley’s work concluded that atomic number is a fundamental property of an atom than atomic weight.
Question 354.
State modern periodic law. How many groups and periods are present in the long form of the periodic table?
Answer:
Modern Periodic law :
“The physical and chemical properties of the elements are periodic functions of their atomic numbers.”
Groups and periods :
The number of groups (vertical columns) in the periodic table = 18
The number of periods (horizontal rows) in the periodic table =7
Question 4.
Why are f-block elements placed below the main table?
Answer:
The two series of f-block elements Lanthanides and Actinides are grouped separately and placed at the bottom of the periodic table, though they belong to the sixth and seventh periods of third group (III B)
This placement is done on the basis of their similar properties. The properties are so similar that elements from Ce to Lu can be considered as equivalent to one element. In case, if these elements are assigned the usual positions, the symmetry’ of the whole arrangement of the periodic table would be disrupted.
Question 5.
Mention the number of elements present in each of the periods in the long form periodic table.
Answer:
Long form periodic table contains 7 periods
Number of elements in the first period = 2
Number of elements in the second period = 8
Number of elements in the third period = 8
Number of elements in the fourth period = 18
Number of elements in the fifth period = 18
Number of elements in the sixth period = 32
Seventh period is an incomplete period and at present, it contains 29 elements.
Question 6.
Give the outer orbit general electronic configuration of
(a) Noble gases
(b) Representative elements
(c) Transition elements
(d) Inner transition elements
Answer:
| Type | General E.C |
| a) Noble gases | ns²np6 (E.C. for He is Is²) |
| b) Representative elements | ns1-2 np0-5 |
| c) Transition elements | (n – 1)d1 – 10 ns1-2 |
| d) Inner transition elements | (n – 2)f1-14(n – 1)d0 – 1ns² |
![]()
Question 7.
Give any four characteristic properties of transition elements.
Answer:
Properties of transition elements :
- They are all hard metals with high B.P and M.P.
- They are good conductors of heat and electricity.
- They form coloured ions.
- They exhibit variable valency oxidation states.
- They possess paramagnetic nature.
- They form complex compounds.
- They act as catalysts.
Question 8.
What are ‘rare earths’ and ‘trans-uranic elements’?
Answer:
Rare earth elements :
The 14 f-block elements in the 4f series, starting from Cerium (Ce) to Lutesium (Lu) are called Rare earth elements, because their abundance in the earth crust is very rare. The properties of all these 14 elements are similar to Lanthanum. So they are also called Lanthanides.
Trans-uranic elements :
The elements of f-block in the 5f series after Uranium (92U) in the periodic table are called transuranic elements. These elements do not occur naturally in the nature. They are man made synthetic elements. They are all radioactive which disintegrate into some other elements.
Question 9.
What is isoelectronic series? Name a series that will be isoelectronic with each of the following atoms or ions.
(a) F– (b) Ar (c) He (d) Rb+
Answer:
A group of atoms or ions having the same number of electrons is called isoelectronic series.
(a) F– relating series :
N-3, O-2, F–, Ne, Na+, Mg+2, Al+3
(b) Ar relating series:
P-3, S-2, Cl–, Ar, K–, Ca+2
(c) ‘He’ relating series:
H–, He, Li+, Be+2
(d) Rb+ relating series:
As-3, Se-2, Br–, Kr, Rb+, Sr+2
Question 10.
Explain why cation is smaller and anion is larger in radii than their parent atoms.
Answer:
When an electron is removed from a neutral atom, cation is formed- The nuclear charge in both the cation and in its parent atom is the same. But the number of electrons in the cation is less than in its parent atom. Hence, the nuclear attractions in a cation will be more than its parent atom. As a result, the electron cloud of cation shrinks. Hence, the size of a cation is always smaller than its parent atom.
When an electron is added to a neutral atom, anion is formed. The nuclear charge in both the anion and in its parent atom is the same. But, the number of electrons in the anion is more than its parent atom. Hence the nuclear attractions, in the anion will be less than its parent atom As a result the electron cloud expands. Hence, the size of anion is always greater than its parent atom.
Question 11.
Arrange the second period elements in the increasing order of their first ionization enthalpies. Explain why Be has higher IEj than B.
Answer:
Li < B < Be < C < O < N < F
Electronic configuration of Be is s²2s².
Electronic configuration of B is 1s²2s²2p¹.
In Berylium, 2s orbital is completely filled and so it is relatively more stable. In Boron, the 2p orbital is partly filled.
In Be, the electron to be removed is from 2s orbital, whereas in B, the electron to be removed is from 2p orbital. The 2s electron of Be is strongly attracted by the nucleus than 2p electron of B. The 2s electron in Be is shielded by only one orbital Is. But 2p electron in B is shielded by two orbitals Is and 2s. So the IE1 of Be is more than IE1 of B.
Question 12.
IE1 of Na is less than that of Mg but IE2 of Na is higher than that of Mg-Explain.
Answer:
Electronic configuration of Na is [Ne]3s¹.
Electronic configuration of Mg is [Ne]3s².
Na atom has only one electron in its outer orbit and by losing that electron it gets stability. Also in the nucleus of Na there are less number of protons than in Mg.
So nuclear attraction on the electrons in Sodium is less than in Magnesium. So IE1 of Na is less than IE1 of Mg.
When an electron in Na+ is lost then the outer orbit of Na+ gets stable octet. To remove one more electron from stable octet, more energy is required. But in Mg+ there is one excess electron to get the stable octet in 3s orbital. To remove that electron, the energy required is less.
So IE2 of Na is greater than IE2 of Mg.
![]()
Question 13.
What are the various factors due to which the IE of the main group elements tends to decrease down a group?
Answer:
1) Atomic size :
With increase in the atomic size down a group, the distance from the nucleus to the outer electrons increases. So the attraction of the nucleus on outer electrons decreases. Hence IE decreases.
2) Nuclear charge :
With increase in nuclear charge i.e., effective nuclear charge, attraction of the nucleus on the outer electrons increases.
So IE increases.
3) Screening effect or Shielding effect:
The inner orbits shield the nuclear attraction on the outer electrons. So with increase in the inner electrons shielding effect increases and thus IE decreases.
4) Extent of penetration of valence shell into inner electron :
The penetrating power of the orbitals towards the nucleus is in the order s > p > d > f. Nuclear attraction on the electrons in these orbitals also will be in the same order. So to remove an electron from different orbitals of the same orbit, the ionisation energy required is in the order s > p > d > f.
5) Number of charges on the ion :
With increase in the number of positive charges on an ion the nuclear attraction on the electrons increases. So IE increases.
6) Electronic Configuration :
Atoms having octet in the outer orbit, or exactly half-filled and completely filled orbitals give stability to the atom. The ionisation energy required for the elements of stable electronic configurations will be more.
Question 14.
The first ionization enthalpy value (in kJ mol-1) of group 13 elements are :

How do you explain the deviation from the general trend?
Answer:
Generally, the I.E1 values decrease from top to bottom, in a group. But in the given data, we observe two deviations.
Deviation 1 (I.E1 of Ga > I.E1 of Al):
Gallium (Ga) has more I.E1 than Aluminium (A I) due to poor shielding effect of completely filled 3d-electrons.
Deviation 2 (I.E1 of Tl > I.E1 of In) :
Thallium (Tl) has more I.E1 than Indium (In) due to poor shielding effect of completely filled 4f-electrons.
Note: B > Tl > Ga > Al > In
Question 15.
Would you expect the second electron gain enthalpy of oxygen as positive more negative or less negative than the first? Justify?
Answer:
The amount of energy released when an electron is added to neutral isolated gaseous atom is called electron gain enthalpy.
When one electron is added to the neutral Oxygen atom it converts into a uninegative ion.
Now, we have to add one more electron to this uninegative ion. But it becomes more difficult to add one more electron to this uninegative ion. Because there arises a repulsion between the negative charge of the ion and negative charge of new electron. Hence some additional energy is needed to overcome these repulsive forces.
So the second electron gain enthalpy of oxygen is always positive.
O + 1e– → O– + energy
O– + 1e– + energy → O-2
Question 16.
What is the basic difference between the electron gain enthalpy and electropositivity?
Answer:
1) Electron gain enthalpy represents tendency of gaining electrons by an isolated atom.
The elements with electron gain enthalpy will have more non-metallic character.
2) Electropositivity represents tendency of losing electrons by an isolated atom.
The elements with electro positivity will have more metallic character.
Question 17.
Would you expect IE1 for two isotopes of the same element to be the same or different? Justify.
Answer:
Two isotopes of the same element have the same number of protons and electrons. Therefore nuclear attraction on the valency electron of two isotopes of the same element is same.
Hence, isotopes of the same element have same I.E values.
Question 18.
Increasing order of reactivity among group-1 elements Li < Na < K < Rb < Cs, whereas among group -17 elements it is F > Cl > Br > l .Explain.
Answer:
The group-1 elements are alkali metals. From top to bottom, in group-1, the atomic radius increases, ionisation energies decrease, electropositive character increases. So the reactivity of alkali metals increases from top to bottom.
F, Cl, Br and I are halogens. They have one electron short of the nearest inert gas configuration. So they have a tendency to gain electron and thus can act as oxidising agents. From top to bottom, in the group, the atomic size increases, electron gain enthalpies decrease. Though electron gain enthalpy of Fluorine is less, it has more electronegativity. Because of high electronegativities than electron gain enthalpies they are highly reactive elements. But, as these values are decreasing from F to I, their reactivity decreases.
![]()
Question 19.
Assign the position of the element ‘ having outer electronic configuration.
a) ns²np4 for n = 3
b) (n – 1) d²ns² for n = 4
Answer:
a) When n = 3, ns²np4 becomes 3s²3p4
Period number = Valence Shell number = 3
Group number = No.of valence electrons = 6
∴ The element is located in the third period and group-6.
b) When n=4, (n – 1) d²ns² becomes 3d²4s²
Period number = Valence Shell number = 4
Group number = Total number of electrons present in 3d²4s² = 2 + 2 = 4
∴ The element is located in the fourth period and group-4.
Question 20.
Predict the formulae of the stable binary compounds that would be formed by the combination of the following pairs of elements.
a) Li and O
b) Mg and N
c) Al and I
d) Si and O
e) P and Cl
f) Element with atomic number 30 & Cl
Answer:
a) Valency of Li is 1 and that of O is 2.
So the formula of compound is Li2O.
b) Valency of Mg is 2 and that of N is 3.
So the formula of compound is Mg3N2.
c) Valency of Al is 3 and that of I is 1.
So the formula of the compound is AlI3.
d) The valency of Si is 4 and that of O is 2.
So the formula of the compound is SiO2.
e) Phosphorous exhibits two types of valencies 3 and 5, but the compound with Cl in +3 oxidation state is stable. So the formula of stable binary compound is PCl3.
f) The element with atomic number 30 is Zinc. Its valency is 2. So the formula of its binary compound with Cl is ZnCl2.
Question 21.
Write a note on the variation of metallic nature in a group and in a period. [AP 18]
Answer:
Metallic nature ∝ Electropositivity
![]()
In a group, from top to bottom, electropositivity increases. So metallic nature also increases.
In a period, from left to right, electronegativity increases and so metallic nature decreases.
Question 22.
How does the covalent radius increase in group 7?
Answer:
The elements of 7th group are Manganese, Technicium and Rhenium.
The covalent radius increases from Manganese to Technicium with increase in the number of orbits.
Technicium atom has 5 orbits while Rhenium contains 6 orbits. Though the number of orbits increases in Rhenium, the covalent radius of Rhenium is almost equal to that of Technicium. This is due to Lanthanide contraction.
Question 23.
Which element of 3rd period has highest IE1? Explain the variation of IE1 in this period.
Answer:
(i) Argon (Ar) of 3rd period has the highest ionization energy.
(ii) The I.E increases across the period due to increase in nuclear charge. Therefore the increasing order of I.E should be
Na < Mg < Al < Si < P < S < Cl < Ar.
But the correct order is
Na < Mg > Al < Si < P > S < Cl < Ar.
This is because of the following anomalies.
(a) E.C of Mg is [Ne]3s²
E.C of Al is [Ne]3s²3p¹
I.E of Mg is more than Al.
Reason :
Mg has completely filled 3s-subshell. Also penetrating power s-orbitals is more than the p-orbital of Al
(b) E.C ofP is [Ne]3s²3p³
E.C of S is [Ne]3s²3p4
I.E of P is more than S.
Reason :
‘P’ has half-filled p-orbitals (3s23p3) and is more stable.
![]()
Question 24.
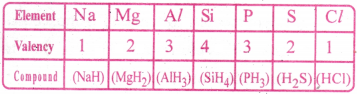
What is valency of an element? How does it vary with respect to hydrogen in the third period.
Answer:
Valency :
The combining capacity of an atom with other atoms is called valency.
It is the number of H atoms (or) the number of Cl atoms (or) double the number of ‘O’ atoms with which one atom of the element combines.
Valency of elements of 3rd period:
Question 25.
What is diagonal relationship? Give a pair of elements having diagonal relationship. Why do they show this relation?
Answer:
Diagonal relationship :
The elements of 2nd period have certain similarities with the elements situated diagonally below in the third period.
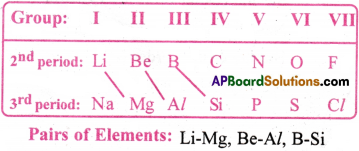
Reasons :
Similar size of atoms, Almost equal electronegative values and polarizing powers of the respective elements.
![]()
Question 26.
What is Lanthanide Contraction? What are its Consequences? [TS 22]
Answer:
The regular decrease of atomic or ionic size from left to right, with increase in atomic number in Lanthanides, is called ‘Lanthanide Contraction’.
In Lanthanides, differentiating electron enters into 4f sub level. Due to peculiar shape and poor shielding effect of 4f-orbitals, the increased nuclear charge attracts valence electrons firmly, causing a steady decrease in the size of atom or ion.
Consequences :
- Due to Lanthanide contraction, the atomic radius of 4d and 5d series elements is almost similar.
Ex: Zr & Hf, Nb & Ta, Mo & W. - The crystal structure and other properties of Lanthanide elements become very close and similar. So it becomes difficult to separate them from a mixture.
- The melting, boiling points and hardness of Lanthanides increase gradually.
- Inert pair effect is consequence of Lanthanide contraction.
- Basic nature of oxides and hydroxides of lanthanides decreases from Ce to Lu.
Long Answer Question
Question 1.
Discuss the classification of elements by Mendeleev.
Answer:
The first, meaningful and remarkable contribution to the classification of elements is done by Mendeleev.
Mendeleev’s Periodic Law :
“The physical and chemical properties of elements are periodic functions of their atomic weights”.
All the elements known at the time of Mendeleev are arranged in the increasing order of their atomic weights. Then the elements having same chemical properties fall into the same vertical columns and the elements whose properties gradually change fall into the same horizontal rows. The vertical columns are named as groups and horizontal rows are named as periods. All together 8 groups and 7 periods are formed.
Periods:
- The periods 1, 2 and 3 are called short periods. They contain 2, 2 and 8 elements respectively.
- The periods 4 and 5 are called long periods. They contain 18 elements each.
- 6th period is the longest period with 32 elements.
- 7th period is an incomplete period with 17 elements.
Groups:
- The groups I to IV are divided into A and B sub groups.
- The VIII group is a peculiar one, with 9 elements arranged in three triads.
They are (Fe, Co, Ni) ; (Ru, Rh, Pd); (Os, Ir, Pt).
Merits of Mendeleev’s table:
1) Mendeleev’s table lead to the development of Modem Periodic table.
2) Discovery of new elements :
Mendeleev left some gaps in his periodic table, for some unknown elements at his time. But he predicted the properties of those unknown elements. Later on, when those elements were discovered, they exactly fitted into those gaps, having properties, predicted by Mendeleev.
Ex : Eka-Boron (Scandium), Eka-Silicon (Germanium), Eka-Aluminium (Gallium) etc.,
3) Placement for ‘Zero’ group elements :
Zero group elements were not known at the time of Mendeleev. Later when they were discovered, they found a proper place in the periodic table under ‘Zero group’.
Demerits of Mendeleev’s periodic table:
1) Position of Hydrogen :
Hydrogen could not be given a proper place, as it resembles both alkali metals and halogens and their properties.
2) Similar elements placed in different groups :
The elements like Ag & T/, Ba & Pb, Cu & Hg show similar properties. But they are placed in different groups.
3) Dissimilar elements placed in same groups :
The elements placed in a particular group of A, B sub groups show quite different properties.
4) Position of Lanthanides and Actinides :
Actually, all these elements are supposed to be placed together in group III. But they are not placed there. This voilates the periodic law.
Question 2.
From a study of properties of neighbouring elements, the properties of an unknown element can be predicted -Justify with an example.
Answer:
From the study of the properties of certain neighbouring elements and their compounds, Mendeleev was able to predict new elements and their properties. Later when those unknown elements were discovered and the predictions were found to be accurate.
| Predicted element | Name of the element |
| Eka-Aluminium | Gallium |
| Eka-Silicon | Germanium |
| Eka-Boron | Scandium |
Zero group elements were not known at the time of Mendeleev. When once the inert gas element Argon was discovered by Rayleigh and Ramsay, the other inert gases of the group are predicted and discovered easily.
Example:
| Properties of Eka-Silicon predicted by Mendeleev (1871) | Properties of Germanium discovered by Winkler (1886) |
| 1) Atomic weight 72 | Atomic weight 72.6 |
| 2) Specific gravity 5.5 | Specific gravity 5.46 |
| 3) Colour-dirty grey | Colour-greyish white |
| 4) Specific heat 0.073 | Specific heat 0.076 |
| 5) Chloride-EsCl4, a liquid | Chloride -GeCl4, a liquid |
| 6) Boiling point below 100°C | Boiling point 86.5°C |
![]()
Question 3.
Discuss the construction of long form of periodic table.
Answer:
Modern periodic law :
‘The physical and chemical properties of elements are the periodic functions of their ‘atomic numbers’.
Long form of periodic table :
In this table all the elements are arranged in the increasing order of atomic numbers. It is a graphical representation of Aufbau’s principle.
Construction :
The table is divided into 7 horizontal rows called periods and 18 vertical columns called groups. Also,the table is divided into 4 blocks.
Periods :
Periods represents principle quantum number of the outer shell.
Each period starts with an alkali metal and ends with a noble gas element.
- The first period contains only 2 elements H and He. Hence it is called shortest period.
- The second period contains 8 elements from Li to Ne. It is called short period.
- The third period also contains 8 elements from Na to Ar. It is also called short period.
- The fourth period contains 18 elements from K to Kr. It is called long period.
- The fifth period contains 18 elements from Rb to Xe. It is also called long period.
- The sixth period contains 32 elements from Cs to Rn. It is called the longest period.
- The seventh period is an incomplete period.lt starts from Fr.
- The 14 Lanthanides and 14 Actinides are placed at the bottom of the table.
- Each period starts with an alkali metal and ends with an inert gas element.
- Most of the physical and chemical properties of elements change gradually in periods.
Groups :
1) All the 18 groups are numbered 1 to 18 according to IUPAC format.
The previous format :
IA( 1), II A( 2), IIIB to VIIB (3 to7), VIII(8, 9, 10), IB( 11), IIB( 12),IIIA to VIIA(13 to 17) and O group! 18)
2) Zero group elements are placed at the extreme right side of the table. They are called noble gas or inert gas elements. They have stable octet configuration.
3) All the elements in a group(family) have same valency. Hence, all the elements in a group show similar properties.
Blocks:
Basing on the entry of the differentiating electron in to subshell of main shell, all the elements are divided in to 4 blocks. They are s-block, p-block, d-block, f-block.
![]()
Question 4.
Discuss the relation between the number of electrons filled into the sub-energy levels of an orbit and the maximum number of elements present in a period.
Answer:
Based on the number of electrons filled into various sub-energy levels of a period, the number of elements in that period can be calculated.
| Period | Sub Energy levels filled | Number of electrons in the period |
| 1st period | 1s | 2 |
| 2nd period | 2s 2p | 8 |
| 3rd period | 3s 3p | 8 |
| 4th period | 4s 3d 4p | 18 |
| 5th period | 5s 4d 5p | 18 |
| 6th period | 6s 4f 5d 6p | 32 |
| 7th period | 7s 5f 6d | Incomplete |
1) First period :
The first period contains’Is’sub shell. It can have a maximum of 2 electrons. Hence the maximum number of elements present in the first period is 2.
2) Second period :
The second period contains ‘2s’ and ‘2p’ subshells. They can have a maximum of 2 and 6 electrons respectively. Hence the maximum number of elements present in the second period is 8.
3) Third period :
The third period contains’3s’and’3p’subshells. They can have a maximum of 2 and 6 electrons respectively. Hence the maximum number of elements present in the third period is 8.
4) Fourth period :
The fourth period contains ‘4s’, ‘3d’ and 4p subshells. They can have a maximum of 2, 10 and 6 electrons respectively. Hence the maximum number of elements present in the fourth period is 18.
5) Fifth period :
The fifth period contains ‘5s’, ‘4d’ and ‘5p’ subshells. They can have a maximum of 2, 10 and 6 electrons respectively. Hence the maximum number of elements present in the fifth period is 18.
6) Sixth period :
The sixth period contains ‘6s’, ‘4f, ‘5d’ and ‘6p’ subshells. They can have a maximum of 2, 14, 10 and 6 electrons respectively. Hence the maximum number of elements present in the sixth period is 32.
7) Seventh period :
The seventh period contains ‘7s’, ‘5f, ‘6d’ and ‘7p’ subshells. They can have a maximum of 2,14,10 and 6electrons respectively. Hence the maximum number of elements present in the seventh period is 32. But actually, this period is incomplete.
Question 5.
Write an essay on s,p,d and f block dements. [Jul’ 01, 02, Mar’05, 11][AP, TS 15,17]
Answer:
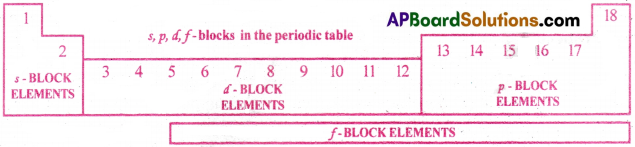
Basing on the entry of differentiating electron into sub-shells of main shells, all the elements are classified into 4 blocks. They are s-block, p-block, d-block, f-block. [AP 20][AP, TS 19]
1) s-block elements:
- The elements in which the differentiating electrons enter into ns-subshell are called s-block elements
- Their general electronic configuration is ns1-2.
- s-block elements are arranged in 2 groups. They are group 1(IA), group 2 (IIA).
- First group (IA) elements are called Alkali metals.
Second group (IIA) elements are called Alkaline earth metals. - s-block is placed on the left side of the periodic table.
2) p-block elements:
- The elements in which the differentiating electrons enter into np-subshell are called p-block elements.
- Their general electronic configuration is ns²np1-6.
- p-block elements are arranged in 6 groups. They are from group 13(IIIA) to group 18.
- p-block starts with 13th group and ends with 18th group.
i) 13th group (or) IIIA group is called Boron family.
ii) 14th group (or) IVA group is called Carbon family.
iii) 15th group (or) VA group is called Nitrogen family.
iv) 16th group (or) VIA group is called Chalcogen family.
v) 17th group (or) VIIA group is called Halogen family.
vi) 18th group (or) 0 group is called Noble gas family. - p-block is placed on the right side of the periodic table.
3) d-block elements:
- The elements in which the differentiating electrons enter into (n-1 )d shell are called d-block elements.
- Their general electronic configuration is (n-1)d1-10 ns1-2.
- d-block elements are arranged in 10 groups.
They are from group 3(IIIB) to group 12(IIB). - d-block elements are further classified into 4 transition series.
They are 3d series, 4d series, 5d series and 6d series. - d-block is placed at the middle of the periodic table.
4) f-block elements :
- The elements in which the differentiating electrons enter into (n-2)f sub shell are called f-block elements.
- Their general electronic configuration is (n-2)f1-14 (n-1)d0-1ns².
- f-block elements are arranged in 14 columns.
- f-block elements are further classified into 2 series.
They are 4f series (Lanthanides). 5f series(Actinides). - f-block is placed separately at the bottom of the periodic table.
Question 6.
Relate the electronic configuration of elements and their properties in the classification of elements.
Answer:
On the basis of electronic configuration and chemical properties, all the elements of the periodic table are divided into 4 types. They are
1) Noble gas elements
2) Representative elements
3) Transition elements
4) Inner transition elements.
Type I : Noble gas elements / Inert gas elements / Rare gases / Aerogens :
- These elements have completely filled outennost shell (ns, np).
- The O group (18th group) elements are called noble gas elements.
They are He, Ne, Ar, Kr, Xe and Rn. - Except for He (1s²), the general outer shell configuration of these elements is ns² np6.
- Properties :
i) All the elements are chemically inactive (under normal conditions).
This is due to the presence of completely filled outer ‘octect’ configuration.
ii) All these elements are mono atomic molecules.
iii) All these are in gaseous state (at ordinary conditions).
Type II : Representative elements / Main group elements :
- Elements in outermost shell in which incompletely filled are called representative elements.
- All the s-block and p-block elements, except group 18 are called representative elements.
- The general outer shell configuration is ns1 or 2 np0 to 5.
- Properties :
i) All these elements are chemically active. This is due to incomplete valence shells. These elements acquire the nearest inert gas configuration by losing (or) gaining (or) sharing electrons.
ii) These elements form ionic and covalent compounds.
iii) All the s-block elements are metals. The p-block elements include metals, non metals and metalloids.
Type III : Transition elements :
- Elements in which n,n-l shells are incompletely filled are called Transition elements.
- All the d-block elements except IIB group are called transition elements.
- The general outer shell configuration is (n-1)d1-10 ns1-2
- Properties :
i) These are hard and heavy metals.
ii) These have high B.P, M.P and density.
iii) These are good conductors of heat and electricity.
iv) These elements show variable valency.
v) These elements exhibit para magnetism.
vi) Most of these act as good catalysts.
vii) These elements form alloys like Brass, Bronze.
viii) These elements form coloured compounds (due to d – d transition).
ix) These elements form complex compounds with other elements.
Type IV : Inner transition elements :
- Elements in which n,(n – 1),(n – 2) shells are incompletely filled are called Innter transition elements.
- The f-block elements are called inner transition elements.
- The general electronic configuration is (n – 2) f1-14 (n-1)do-1ns².
- Properties :
i) These are metals with high B.P and M.P.
ii) These elements show variable oxidation states.
iii) These elements exhibit para magnetism.
iv) These elements form coloured compounds.
v) These elements form complex conpounds.
Question 7.
What is periodic property? How the following properties vary in a group and a period? Explain (I) Atomic radius (2) Electron gain enthalpy [AP 15][IPE’ 11, 14, 14][TS 15, 16, 18]
Answer:
Periodic property :
In the period table, some properties of elements change gradually with a change in their electronic configurations. Such properties are called periodic properties.
1) Atomic radius :
The distance between the centre of the atomic nucleus and the electron cloud of the outer most energy level is called atomic radius.
i) In a group, from top to bottom, the atomic radius increases.
Reason :
In a group, the differentiating electron enters the next orbit.
Hence atomic radius increases
ii) In a period, from left to right, the atomic radius decreases.
Reason :
In a period, the differentiating electron remains in the same orbit. Hence atomic radius decreases.
2) Electron gain enthalpy :
The amount of energy released when an electron is added to neutral isolated gaseous atom is called electron gain enthalpy (or) electron affinity(EA).
i) In a group, from top to bottom, electron gain enthalpy decreases.
Reason:
In a group, the atomic size increases.
Hence, the effective nuclear attraction on outer electrons decreases.
Thus E.A decreases from top to bottom in a group.
ii) In a period, from left to right, the electron gain enthalpy increases.
Reason:
In a period, the atomic size decreases.
Hence, the effective nuclear attraction on outer electrons increases.
Thus electron affinity increases from left to right in a period.
Question 8.
What is periodic property? How the following properties vary in a group and a period? Explain(l) Ionisation potential (2) Electro negativity [AP, TS 18][IPE’ 10, 11, 14, 14][AP, TS 15, 16]
Answer:
Periodic property :
In the period table, some properties of elements change gradually with ‘ a change in their electronic configurations. Such properties are called periodic properties.
1) Ionisation Potential(IP) :
The minimum energy required to remove an electron from the outer most valence shell from an isolated, neutral, gaseous atom is called ionisation energy.
i) In a group, from top to bottom, the I.P. value decreases.
Reason:
In a group, the atomic size increases.
Hence, the effective nuclear attraction on outer electrons decreases.
Thus I.P. value decreases from top to bottom in a group.
ii) In a period, from left to right, the I.P value increases.
Reason:
In a period, the atomic size decreases.
Hence, the effective nuclear attraction on outer electrons increases.
Thus I.P. value increases from left to right in a period.
2) Electronegativity(EN) :
The tendency of an atom in a molecule to attract the shared pair of electrons towards itself is called electronegativity.
i) In a group, from top to bottom, the E.N. value decreases.
Reason:
In a group, the atomic size increases.
Hence, the effective nuclear attraction on outer electrons decreases.
Thus E.N value decreases from top to bottom in a group.
ii) In a period, from left to right, the E.N value increases
Reason:
In a period, the atomic size decreases.
Hence, the effective nuclear attraction on outer electrons increases.
Thus E.N value increases from left to right in a period.
Question 9.
Write a note on
a) Atomic radius
b) Metallic radius
c) Covalent radius
Answer:
a) Atomic radius :
The distance between the nucleus and outermost electron of an atom is called atomic radius.
As the atomic radius increases, the distance between the nucleus and the outer most electrons increases. Hence, the effective nuclear charge on the outermost electrons decreases. As a result, the energy required to remove the electrons decreases. Hence we conclude that, as atomic radius increases, I.P decreases and vice versa.
Ex: Atomic radius of Na = 186pm
b) Metallic radius :
The half of the inter nuclear distance between two adjacent atoms in a metallic crystal is called metallic radius.
Ex : The inter nuclear distance between two Sodium atoms is 3.72 Å.
∴ Metallic radius of Sodium = \(\frac{3.72}{2}\) 1 .o6Å
c) Covalent radius :
The half of the inter-nuclear distance between two adjacent atoms of a covalent molecule is called covalent radius.
Ex : The inter-nuclear distance between Chlorine atoms in C^ molecule is 1.98 Å
∴ Covalent radius of Chlorine = \(\frac{1.98}{2}\) 0.99 Å
![]()
Question 10.
Define IE1 and IE2. Why is IE2 > IE1 for a given atom? Discuss the factors that effect IE of an element. [AP 16, 17, 18, 19, 22][Mar’13, May’13][TS-16, 17, 19, 22]
Answer:
1) First ionisation enthalpy (IE1):
The minimum energy required to remove an electron from the outer most shell of an isolated, neutral, gaseous atom is called first ionisation enthalpy.
M(g) + I.E1 → M+(g) + e–
Second ionisation enthalpy (IE2) :
The minimum energy required to remove an electron from a unipositive gaseous ion is called second ionisation enthalpy.
M+(g) + I.E2 → M+2 (g) + e–
Second ionisation enthalpy (IE2) is greater than first ionisation enthalpy (IE1).
Reason :
In a neutral atom, the number of electrons is equal to number of protons. But, in a uni-positive ion, the number of protons is greater than the number of electrons. So, nucleus of unipositive ion attracts the outer electrons with more force than the nucleus of its neutral atom. So the second ionization enthalpy is greater than the first ionization enthalpy.
3) Factors affecting the ionisation enthalpy:
i) Atomic radius :
When atomic radius increases, the nuclear force of attraction on the valence electrons decreases. So, I.E value also decreases.
ii) Nuclear charge :
When the nuclear charge increases, the force of attraction on the valence electron increases. So, I.E value also increases.
iii) Screening effect :
The electrons present in the ‘inner orbits’ decrease the nuclear attractions between nucleus and the outer electrons. This is1 known as screening effect.
When the number of inner shells increases, the attraction of nucleus on the outer electrons decreases. So, the I.E value also decreases.
iv) Penetrating effect :
In a given shell, the penetrating power of the valence electrons decreases in the order of s > p > d > f. So, ‘ns’ electrons are more tightly held by the nucleus. So, the I.E value decreases in the same order.
v) Completely filled (or) half-filled sub-shells :
Atoms with completely filled (or) half-filled sub-shells are more stable than the others.
Such elements have slightly higher I.E values than excepted.
Question 11.
How do the following properties change in group-1 and in the third period?
Explain with example, a) Atomic radius b) IE C) EA d) Nature of oxides.
Answer:
The elements of Group IA : Li, Na. K, Rb. Cs (These elements are called Alkali metals)
The elements of third period : Na, Mg, A/, Si, P, S, Cl, Ar.
a) Atomic radius:
IA group elements :
The atomic radii of elements of group IA increase gradually due to the increase in the number of shells from top to bottom. The atoms of these elements have the largest size in their corresponding periods.
Order of atomic radius of IA group elements: Li < Na < K < Rb < Cs
3rd period elements :
The atomic radius decrease from left to right across the 3rd period. This is due to increase in the effective nuclear charge.
The first element Na has largest size and Chlorine has smallest size. But the size of last element Argon is larger than its preceeding element Chlorine because Ar is measured in Vanderwalfs radius.
Order of atomic radius of 3rd period elements: Na > Mg > Al > Si > P > S > Cl < Ar
b) Ionisation Potential:
IA group elements: The values of IE1 decrease down the group from Li to Cs. This is due to increase in their atomic radii.
3rd period elements :
The values of IE1 increase from Na to Ar with some exceptions.
IE1 of Mg(3s²) is more than Al(3s²3p²). This is due to completely filled s orbitals of Mg
IE1 of P(3s²3p³) is more than S(3s²3p4). This is due to half-filled p orbitals of Phosphorus.
Order of IE1 in 3rd period elements : Na < Mg > Al < Si < P > S < Cl < Ar
c) Electron affinity(EA) :
IA Group elements :
In IA group, from top to bottom, the size and nuclear charge increase. But the effect of increase in atomic size is much more pronounced than that of nuclear charge. Thus the additional electron feels less attraction by the large atom. Consequently electron affinity is less negative. Hence electron affinity decreases from Li to Cs. Order of EA in IA Group elements : Li > Na > K > Rb > Cs
3rd period :
In 3rd period, from left to right, the size of the atom decreases and nuclear charge increases. Hence electron affinity becomes more negative in this period from Na to Cl.
d) Nature of oxides:
IA group elements :
The oxides of Alkali metals Li2O, Na2O, K2O, Rb2O, Cs2O are basic in nature. Their basic nature increases down the group from Li2O to Cs2O.
3rd period elements :
The oxides of 3rd period elements are Na2O, MgO, Al2O3, SiO2, P2O10, SO3 and Cl2O7. The basic nature of these oxides decreases and acidic nature increases from left to right.

Question 12.
Define electron gain enthalpy. How it varies in a group and in a period? Why is the electron gain enthalpy of O or F is less negative than that of the succeeding clement ia the group?
Answer:
a) Electron gain enthalpy(∆egH) :
When an electron is added to a neutral gaseous atom [X] to convert it into a negative ion, the enthalpy change accompanying in this process is defined as the Electron gain enthalpy.
Depending on the element, the process of adding an electron to the atom, can either be Endothermic (or) Exothermic.
Variation in a group :
In a group, from top to bottom, electron gain enthalpy becomes less negative.
Reason :
It is due to increase in atomic size and screening effect.
Variation in a period :
In a period from left to right, electron gain enthalpy becomes more negative
Reason :
It is due to decrease in atomic size, increase of effective nuclear charge.
b) Electron gain enthalpy of O or Fis less than that of their succeeding elements S or Cl
Reason : E.C of Oxygen : 1s²2s²2p4
E.C of Sulphur; 1s²2s²2p63s²3p4
E.C of Fluorine : 2s²2s²2p5
E.C of Chlorine: 1s²2s²2p63s²3p5
Explanation :
In Oxygen or Fluorine, the added electron goes to the smaller quantum level n = 2. Due to small size, it suffers significant repulsion from the other electrons present in this level. So energy released will be less.
For succeeding elements S, Cl the added electron goes to quantum level n = 3. Due to large size electron-electron repulsion is much less. So the E. A of succeeding element will be more.
![]()
Question 13.
a) What is electronegativity? b) How does it vary in a group and in a period?
Answer:
Electronegativity :
“The tendency of an atom to attract the shared electron pair, more towards itself, in a di-atomic molecule is called electronegativity”.
Variation of Electronegativity in a group :
In a group, from top to bottom, the values of electronegativity decrease.
Reason:
- In a group, from top to bottom, there is an increase in the size of the atoms. With the increase in size of the atoms, their electronegativity values decrease.
- Ionization potential and electron affinity decrease from top to bottom, in a group.
Hence electronegativity also decreases from top bottom, in a group.
Varialion of electronegativity in a period :
In a period, from left to right , the values of electronegativity increase. (‘F’ is most electronegative element)
Reason:
- In a period, from left to right, there is decrease in the size of the atoms. Smaller atoms have greater tendency to attract the electrons towards themselves.Thus the electronegativity values increase from left to right, in a period.
- In a period, from left to right, there is an increase of ionization potential and electron affinity of the elements. The atoms of the elements which have higher values of ionization energy and electron affinity also have higher Electronegativity values.
Question 14.
Explain the following:
(a) Valency (b) Diagonal relation (c) Variation of nature of oxides in the Group-1
Answer:
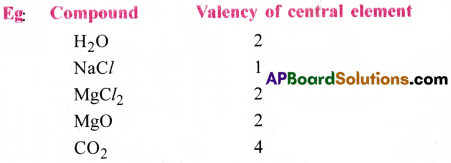
(a) Valency :
The combining capacity of an atom with other atoms is called valency.
It is the number of H atoms (or) the number of Cl atoms (or) double the number of ‘O’ atoms with which one atom of the element combines.
The number of electrons present in the outermost shell of an atom is called its valency. Valency of an element is the number of electrons gained or lost or shared with other atom in the formation of a compound. The valency of an element is useful in writing the formulae of compounds. Valency is always a whole number.
∴ Valency = No. of hydrogens in its compounds
= No. of chlorine atoms in its compounds
= 2 × no. of oxygen atoms present in the molecule.
Variation of valency in a period :
In a period, the valency of an element increases from left to right, due to increase of number of outermost electrons.
The valency w.r.t Hydrogen increases from 1 to 4 and then decreases to 1, from group IA to group VIIA, of a period. The valency w.r.t Oxygen increases from 1 to 7 in a period.
Variation of Valency in a group:
From IA to IVA groups, the valency of an element is equal to its group number.
From VA to VIIA groups, the valency of an element is equal to 8- group number.
Valency of noble gases is ‘zero’ due to the presence of completely filled configuration (ns²np6)
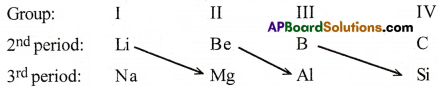
(b) Diagonal relationship :
The elements of 2nd period have certain similarities with the elements situated diagonally below in the third period. This is called diagonal relationship.
Eg: (Li-Mg); (Be-Al); (B-Si)
(c) Variation of nature of oxides in the Group-1 :
IA group elements are called alkali metals. Alkali metals form M2O type monoxides. They are Li2O, Na2O, K2O, Rb2O, Cs2O. Generally, oxides of metals are basic in nature. So, oxides of alkali metals are basic in nature.
As we move from top to bottom in a group the metallic character increases. Flence the basic nature of oxides increases from Li2O to Cs2O.
Order of basic nature of oxides of alkali metals: Li2O < Na2O < K2O < Rb2O < Cs2O.
Multiple Choice Questions
Question 1.
The period number in the long form of the periodic table is equal to
1) magnetic quantum number of any element of the period.
2) atomic number of any element of the period.
3) maximum Principal quantum number of any element of the period.
4) maximum Azimuthal quantum number of any element of the period.
Answer:
3) maximum Principal quantum number of any element of the period.
![]()
Question 2.
The elements in which electrons are progressively filled in 4f-orbital are called
1) actinoids
2) transition elements
3) lanthanoids
4) halogens
Answer:
3) lanthanoids
Question 3.
Consider the isoelectronic species, Na+, Mg2+, F– and O2-. The correct order of increasing length of their radii is
1) F– < O2- < Mg2+ < Na–
2) Mg2+ < Na+ < F– < O2-
3) O2- < F– < Na+ < Mg2+
4) O2- < F– < Mg2+ < Na+
Answer:
2) Mg2+ < Na+ < F– < O2-
Question 4.
The first ionisation enthalpies of Na, Mg, Al and Si are in the order:
1) Na < Mg > Al < Si 2) Na > Mg > Al > Si
3) Na < Mg < Al < Si 4) Na > Mg > Al < Si
Answer:
1) Na < Mg > Al < Si
Question 5.
With which of the following electronic configuration an atom has the lowest ionisation enthalpy?
1) 1s² 2s² 2p³
2) 1s² 2s² 2p5 3s¹
3) 1s² 2s² 2p6
4) 1s² 2s² 2p5
Answer:
2) 1s² 2s² 2p5 3s¹
Question 6.
The order of screening effect of electrons of s, p, d and f orbitals of a given shell of an atom on its outer shell electrons is:
1) s > p > d > f
2) f > d > p > s
3) p < d < s > f
4) f > p > s > d
Answer:
1) s > p > d > f
Question 7.
For the second-period elements the correct increasing order of first ionization enthalpy is
1) Li < Be < B < C < O < N < F < Ne
2) Li < Be < B < C < N < O < F < Ne
3) Li < B < Be < C < O < N < F < Ne
4) Li < B < Be < C < N < O < F < Ne
Answer:
3) Li < B < Be < C < O < N < F < Ne
![]()
Question 8.
Electronic configurations of four elements A, B, C and D are given below:
(A) 1s² 2s² 2p6
(B) 1s² 2s² 2p4
(C) 1s² 2s² 2p6 3s¹
(D) 1s² 2s² 2p5
Which of the following is the correct order of increasing tendency to gain electron :
1) A < C < B < D
2) A < B < C < D
3) D < B < C < A
4) D < A < B < C
Answer:
1) A < C < B < D
Question 9.
What is the value of electron gain enthalpy of Na+ if IE1 of Na = 5.1 eV?
1) -5.1 eV
2) -10.2 eV
3) +2.55 eV
4) +10.2eV
Answer:
1) -5.1 eV
Question 10.
Among halogens, the correct order of amount of energy released in electron gain (electron gain enthalpy) is:
1) F > Cl > Br > I
2) F < Cl < Br < I
3) F < Cl > Br > I
4) F < Cl < Br < I
Answer:
3) F < Cl > Br > I
Question 11.
Which of the following oxides is amphoteric?
1) SnO2
2) CaO
3) SiO2
4) CO2
Answer:
1) SnO2
Question 12.
Which of the following orders of ionic radii is correctly represented?
1) H– > H+ > H
2) Na+ > F– > O2-
3) F– > O2- > Na+
4) Al3+ > Mg2+ > N3-
Answer:
1) H– > H+ > H
![]()
Question 13.
Which of the following is the correct order of size of the given species:
1) I > I– > I+
2) I+ > I– > I
3) I > I+ > I–
4) I– > I > I+
Answer:
4) I– > I > I+
Question 14.
The electronic configuration of gadolinium (Atomic number 64) is
1) [Xe] 4f³ 5d5 6s²
2) [Xe] 4f7 5d² 6s¹
3) [Xe] 4f7 5d¹ 6s²
4) [Xe] 4f8 5d6 6s²
Answer:
3) [Xe] 4f7 5d¹ 6s²
Question 15.
The element Z = 114 has been discovered recently. It will belong to which of the following family/group and electronic configuration?
1) Carbon family, [Rn]5f146d107s²7p²
2) Oxygen family, [Rn]5f146d107s²7p4
3) Nitrogen family, [Rn]5f146d107s²7p6
4) Halogen family, [Rn]5f146d107s²7p5
Answer:
1) Carbon family, [Rn]5f146d107s²7p²
Question 16.
Match the oxide given in column I with its property given in column II.
| Column I | Column II |
| i) Na2O | A) Neutral |
| ii) Al2O3 | B) Basic |
| iii) N2O | C) Acidic |
| iv) Cl2O7 | D) Amphoteric |
Which of the following options has all correct pairs?
1) (i)-B, (ii)-A, (iii)-D, (iv)-C
2) (i)-C, (ii)-B, (iii)-A, (iv)-D
3) (i)-A, (ii)-D, (iii)-B, (iv)-C
4) (i)-B, (ii)-D, (iii)-A, (iv)-C
Answer:
4) (i)-B, (ii)-D, (iii)-A, (iv)-C
![]()
Question 17.
Which of the following oxides is most acidic in nature?
1) MgO
2) BeO
3) BaO
4) CaO
Answer:
2) BeO
Question 18.
Which of the following is the most basic oxide?
1) SeO2
2) Al2O3
3) Sb2O3
4) Bi2O3
Answer:
4) Bi2O3
Question 19.
Which of the following is not an actinoid?
1) Curium (Z = 96)
2) Californium (Z = 98)
3) Uranium (Z = 92)
4) Terbium (Z = 65)
Answer:
4) Terbium (Z = 65)
![]()
Question 20.
Identify the incorrect match.
| Name | IUPAC Official Name |
| (A) Unnilunium | (i) Mendelevium |
| (B) Unniltrium | (ii) Lawrencium |
| (C) Unnilhexium | (iii) Seaborgium |
| (D) Unuinunnium | (iv) Darmstadtium |
1) (A), (i)
2) (B), (ii)
3) (C), (iii)
4) (D), (iv)
Answer:
4) (D), (iv)