Students get through AP Inter 1st Year Chemistry Important Questions 3rd Lesson Chemical Bonding and Molecular Structure which are most likely to be asked in the exam.
AP Inter 1st Year Chemistry Important Questions 3rd Lesson Chemical Bonding and Molecular Structure
Very Short Answer Questions
Question 1.
What is Octet rule? [TS 22]
Answer:
Octet rule :
Atoms show a tendency, to have eight electrons in their outermost shell by losing, gaining or sharing electrons.
Question 2.
Write Lewis dot structures for S and S2-
Answer:

Question 3.
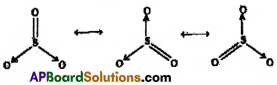
Write the possible resonance structures for SO3.
Answer:
Resonance structures of SO3:
Question 4.
Predict the change, if any, in hybridisation of Al atom in the following reaction
AlCl3 + Cl– + AlCl4–
Answer:
In AlCl3, Aluminium undergoes sp² hybridisation. In AlCl4–, Aluminium undergoes sp³ hybridisation.
So, in the given reaction, hybridisation of Al changes from sp² to sp³.
Question 5.
Which of the two cations Ca2+ or Zn2+ is more stable and why? [TS 22]
Answer:
Ca2+ ion is more stable than Zn2+.
Ca2+(2, 8, 8) has inert gas configuration while Zn2+ (2, 8, 18) has pseudo inert gas configuration.
Reason :
An atom with inert gas configuration is more stable than an ion with pseudo inert gas configuration.
![]()
Question 6.
Chloride ion has greater stability than Chlorine atom. Why?
Answer:
Cl( 17) atom has one electron short to the nearest inert gas (Ar) configuration.
Hence it is unstable.
But Cl– has Ar (18) configuration.
Hence it is stable.
Question 7.
Why is a diatomic molecule of argon (Ar2) is not formed?
Answer:
‘Ar’ atom contains paired electrons with stable octet configuration. Thus, it can’t share its electrons with another Ar atom. Hence, Ar does not form a diatomic molecule.
Question 8.
What is the best possible arrangements of four bond pairs in the valence shell of an atom to minimize repulsions?
Answer:
The best possible arrangement to minimize repulsions with four bond pairs in valency shell of an atom is Tetrahedral with bond angle 109°28′.
Question 9.
If A and B are two different atoms when does AB molecule become covalent?
Answer:
- The difference between the electronegative values of A and B should be less than 1.7
- A and B should share one or more electron pairs mutually.
Then A and B form a covalent molecule.
Question 10.
What is meant by localized orbitals?
Answer:
The molecular orbital with localized ‘bonded electron cloud’ between the two nuclei of bonded atoms is called localized orbital.
![]()
Question 11.
How many σ and π bonds are present in (a) C2H2 (b) C2H4? [AP-15][TS-22]
Answer:
a) Structure of acetylene (C2H2) is
H-C≡C-H. It contains three σ bonds and two π bonds.
b) Structure of ethylene (C2H4) is
H2C=CH2
It contains five σ bonds and one π bond.
Question 12.
Is there any change in the hybridisation of Boron and Nitrogen atom as a result of the following reaction? [Imp.Q]
BF3 + NH3 → F3BNH3
Answer:
- The hybridisation of Boron before the reaction is sp² and after the reaction is sp³.
- The hybridisation of Nitrogen before and after the reaction is sp³
Short Answer Questions
Question 1.
Explain Kossel-Lewis approach to Chemical bonding.
Answer:
Kossel-Lewis approach gave a logical explanation to the fonnation two types of chemical bonds.
According to them, atoms combine to acquire nearest inert gas configuration by transferring or sharing of valence electrons.
Kossel’s approach :
A chemical bond can be formed by the transfer of valence electrons from one atom to another.
Kossel proposed that the highly electronegative elements like Halogens, gain electrons and convert into anions. The highly electropositive alkali metals lose electrons and convert into positive ions.
During their conversion into ions, they get the noble gas octet configuration. Now the positive and negative ions unite together by electrostatic attraction between them. Thus Kossel proposed the ionic bond formation.
Lewis approach :
A chemical bond can be formed by the mutual sharing of valence electrons.
Lewis considered the atom as a positively charged ‘Kernel’ (Kernel consists of inner electrons and nucleus) Lewis assumed that the outer shell can accommodate a maximum of eight electrons which occupy the eight comers of a cube surrounding the Kernel. He assumed that noble gases are stable due to this type of arrangement. The atoms which do not have this type of arrangement achieve the stable octet, by sharing of electrons to form chemical bonds.
Thus, Lewis proposed the covalent bond formation and Kossel proposed ionic bond fonnation.
Question 2.
Write the general properties of Ionic compounds.
Answer:
1) Physical state :
In general, all ionic substances are crystalline solids.
2) Reactivity :
Reactions between ionic compounds are very fast in aqueous solutions.
3) Isomerism :
Ionic bond is non directional. So, they do not exhibit isomerism.
4) Solubility :
Ionic compounds are soluble in polar solvents like water, liquid ammonia.
5) Electrical conductivity :
Ionic substances conduct electricity in molten state /aqueous solution.
6) Melting and boiling points :
They possess high B.P and M.P.
Memory Tip : Remember PRISEM
Question 3.
State Fajan’s rules and give suitable examples. [AP 15,16,19,22]
Answer:
Fajan’s rules are useful to predict the nature of the chemical bond in a molecule.
1) As the size of cation increases, the ionic nature of the bond increases.
Ex: Among alkali metal ions the ionic nature of the bond is in the order Li+ < Na+ < K+ < Rb+
2) Smaller the size of the anion, greater is its ionic nature.
Ex : Among CaF2 & Cal2, CaF2 is more ionic, as the size of F– ion is smaller than that of I– ion.
3) Low charges on cation (or) anion (or) both the ions, favour the formation of ionic bond.
Ex: NaCl is more ionic than AlCl3 as the charge on Na (Na+) is less than that of Al (Al+3).
4) Cations with inert gas configuration form ionic compounds, while cations with pseudo inert gas configuration favour covalent bond formation.
Ex: NaCl is ionic, since Na+(2, 8) has inert gas configuration.
CuCl is more covalent, since Cu+ (2, 8, 18) has pseudo inert gas configuration.
Question 4.
What is Octet rule? Briefly explain its significance and limitations.
Answer:
Octet Rule :
“Atoms show tendency to have 8 electrons in their outermost shell”.
The octet configuration is attained by losing or gaining or sharing of electrons between two atoms.
Significance:
- It is the basis of electronic theory of valency.
- It explains the chemical inactivity of zero group elements.
- It is useful for understanding the structure of most of organic compounds.
Limitations:
- Octet rule couldn’t explain the shapes of molecules.
- Octet rule is not satisfied for molecules having odd number of electrons.
Ex: NO, NO2 - It could not explain the stability of molecules in which central atom has less or more than eight electrons.
Ex: In BeCl2, Be has 4 electrons in its valence shell.
In PCl5, P has 10 electrons in its valence shell.
![]()
Question 5.
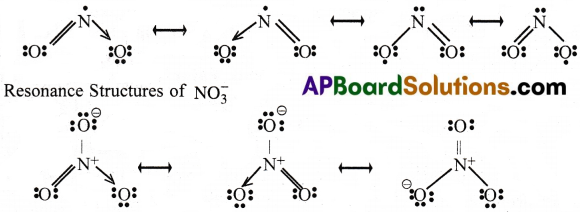
Write the resonance structures for NO2 and NO3.
Answer:
Resonance Structures of NO2.
Question 6.
Use Lewis symbols to show electron transfer between the following pairs of atoms to form cations and anions: (a) K and S ‘(b) Ca and O (c) Al and N.
Answer:
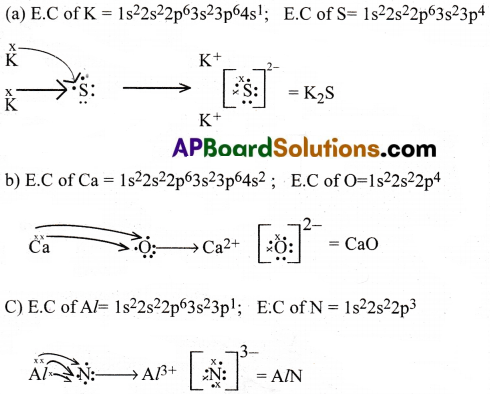
Question 7.
Explain why H2O has dipole moment while CO2 does not have?
Answer:
Dipole moment of H2O is positive :
Reasons:
1) H2O is a polar molecule with 2 polar bonds.

3) The two moments of O-H bonds donot cancel each other, as they are not exactly in opposite directions.
∴ Net dipole moment becomes positive.
Dipole moment of CO2 is zero :
![]()
Reasons :
1) CO2 is a non-polar molecule with two polar bonds.
2) CO2 has a ‘linear structure’.
3) The two moments of C=0 bonds cancel each other, as they are exactly in opposite directions.
∴ Net dipole moment becomes zero.
Question 8.
Define dipole moment. Write its applications. [AP 20]
Answer:
Dipole moment (µ):
The product of the magnitude of the charge(Q) on either of the poles and the distance(r) between the centres of positive and negative charges is called dipole moment.
Mathematically, µ = Q × r
Where µ = Dipole moment; Q= charge;
r = distance between the charges.
Units: D (Debye units).
Applications:
- Dipole moment predicts the polarity of molecules.
a) Molecules with dipole moment greater than zero are polar.
b) Molecules with dipole moment is equal to zero are non polar. - It predicts the shape of the molecules.
- It predicts the % ionic character of covalent bonds.
% ionic character of a covalent bond

Question 9.
Explain why BeF2, molecule has zero dipole moment, although the Be-F bonds are polar.
Answer:
- BeF2 is a non polar molecule, with two polar bonds.
- BeF2 has a ‘linear structure’.
- The two moments of Be-F bonds cancel each other, as they are exactly in opposite directions.
∴ Net dipole moment becomes zero.
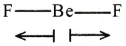
Question 10.
Explain the structure of CH4 molecule. [AP 18]
Answer:
Structure of CH4 (Methane) :
- The central atom of CH4 is Carbon.
- Atomic number of C is 6.
Its ground state E.C = 1s²2s²2p²
Its Excited state E.C = 1s²2s¹2p¹x2p¹y2p¹z - In its excited state, the central Carbon atom undergoes sp³ hybridisation
- One 2s orbital and three 2p orbitals of Carbon, form 4 sp³ hybrid orbitals.
- Now, these 4 hybrid orbitals overlap in a head-on position with s- orbital of four H atoms and they form 4 σ bonds.
- The structure of CH4 is Tetrahedral. The bond angle is 109°28′.
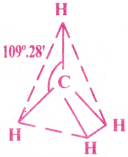
Since there are no lone pairs, there is no distortion in the shape of the molecule.
Question 11.
Explain Polar Covalent bond with a suitable example.
Answer:
Polar Cpvalent Bond :
A covalent bond formed between two non-metal atoms of different elements is called Polar Covalent bond.
In these bonds, the electrons are shared unequally and the bonded atoms acquire a partial positive charge and negative charge.
Ex : The bond in HF is Polar Covalent bond.
Expianation :
Since Fluorine has high electronegativity, the electronegativity of Fluorine is greater than that of hydrogen. Thus in HF bond, the Fluorine end is negative whereas hydrogen end is positive. Hence the bond in HF is a Polar Covalent bond.
![]()
Question 12.
Explain the shape and bond angle in BCl3 molecule in terms of Valence Bond Theory.
Answer:
Structure of BCl3 :
- The central atom of BC/3 is Boron.
- Atomic number of B is 5.
Its ground state E.C = 1s²2s²2p¹
Its Excited state E.C = 1s²2s¹2p¹x2p¹z2p0z - In its excited state, the central Boron atom undergoes sp² hybridisation.
- One 2s orbital and two 2p orbitals of Boron, form three sp² hybrid orbitals.
- Now, the three sp² hybrid orbitals of’B’ atom overlap axially with 3pz orbitals of three Cl atoms, and they form three σ bonds.
- The shape of BCl3 is plane Triangular, with bond angle 120°.
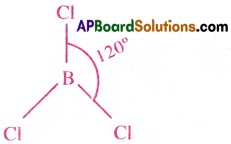
Question 13.
What are σ -and π bonds? Specify the differences between them.
Answer:
| σ – bond | π – bond |
| 1) σ-bond is a covalent bond formed by the axial overlapping of orbitals. | 1) π -bond is a covalent bond formed by the side wise overlapping of orbitals. |
| 2) σ -bond is a strong bond. | 2) π -bond is a weak bond. |
| 3) It allows free rotation of atoms. | 3) It restricts the free rotation of atoms. |
| 4) σ -bonds determine the shape of the molecules. | 4) The shape of the molecules is not influenced by π bonds |
| 5) It forms independently. | 5) π bond always follows σ-bond. |
| 6) There can be only one σ- bond between two atoms. | 6) There can be one or two π – bonds between two atoms. |
![]()
Question 14.
Eventhough nitrogen in ammonia is in sp³ hybridisation, the bond angle deviates from 109“28′. Explain.
Answer:
The central atom of NH3 molecule is N. It undergoes sp³ hybridisation. It contains one lone pair and three bond pairs of electrons. So its shape should be Tetrahedral with bond angle 109°28′. But according to VSEPR theory, repulsions arise between lone pair and bond pair of electrons. So the bond angle reduces to 107° and molecular shape changes to Pyramidal.
Question 15.
Show how a double and triple bond are formed between carbon atoms in
(a) C2H4 and (b) C2H4 espectively. [AP 19]
Answer:
(a) C2H4 (Ethylene):
- In C2H4, the two carbon atoms undergoes sp² hybridisation.
- They both form six sp²hybrid orbitals.
- At first, one sp² hybrid orbital of one C atom overlaps axially with sp² orbital of other C atom and forms one C-C σ bond.
- The remaining four sp² hybrid orbitals overlap axially with s- orbitals of four ‘H’ atoms and they form four σ bonds.
- Now, each C atom is left with one pure p-orbital. They overlap each other laterally and form one π bond.
- Thus,a double bond(one σ and one π bond) is formed in between the two C atoms.
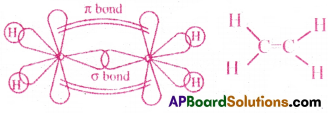
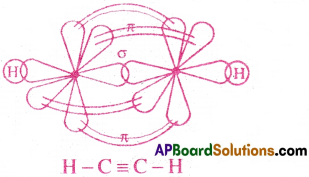
(b) C2H2 (Acetylene)
- In C2H2, the two carbon atoms undergo sp hybridisation.
- They both form 4 sp hybrid orbitals.
- At first, one sp hybrid orbital of one C atom overlaps axially with sp orbital of other C atom and forms one C-C σ bond.
- The remaining 2 sp hybrid orbitals overlap axially with s- orbitals of two ‘H’ atoms and they form two σ bonds.
- Now, each C atom is left with two pure p-orbitals. They overlap each other laterally and form two π bonds.
- Thus, a triple bond(one σ and two π bonds) is formed between the two C atoms.
Question 16.
Explain the hybridisation involved in PCl5 molecule. [AP 18, 20, 22][TS 15, 16, 18, 20]
Answer:
- The central atom of PCl5 is Phosphorus
- Atomic number of P is 15.
Its ground state E.C= [Ne]3s²3p³
Its Excited state E.C= [Ne] 3s¹3p¹x 3p¹y 3p¹z, 3d¹ - In its excited state the central P atom undergoes sp3d hybridisation.
- It forms five sp³d hybrid orbitals.
- The five ‘sp³d’ hybrid orbitals of P, overlap axially with 3pz orbital of five Cl atoms and they form five strong sp³d a bonds.
- The shape of PCl5 molecule is Trigonal bipyramid with bond angles 120°,90°,180°.
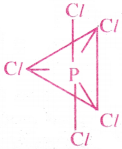
Question 17.
Explain the hybridisation involved in SF6 molecule. [AP, TS 19]
Answer:
- The central atom of SF6 is Sulphur.
- Atomic number of S is 16.
Its ground state E.C= [Ne]3s²3p4
Its Excited state E.C
= [Ne] 3s¹3p¹x 3p¹y 3p¹z 3d¹(x² – y²) 3d¹z² - In its excited state the central S atom undergoes sp³d² hybridisation.
- It forms six sp³d² hybrid orbitals.
- The 6 sp³d² hybrid orbitals of S overlap axially with p orbital of six F atoms and they form six sp3d2 sigma(a) bonds.
- The shape of SF^ molecule is Octahedral with bond angles 90‘ and 180°.
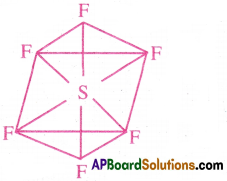
Question 18.
Explain the formation of Coordinate Covalent bond with one example. [TS 19][AP 16]
Answer:
Co-ordinate covalent bond is a special type of covalent bond. In this type, two atoms share one pair of electrons, but the shared pair is contributed by only one of the atoms.
The atom which contributes the electron pair for sharing is called donor atom, while the other atom which accepts the electron pair for sharing is called acceptor atom.
The donor atom must be having one or two lone pairs of electrons, while the acceptor atom is short of two electrons to get its octet. The Co-ordinate bond is represented by an arrow mark from donor to acceptor (A → B)
Formation of ammonium ion (NH+4):
Ammonium ion is formed by the union of NH3 molecule with H+ ion. In NH3 molecule, the central ‘N’ atom has one lone pair of electrons and H+ ion has empty orbital. Flence, N atom of NH3 molecule donates its lone pair to the empty Orbital of H+ion. Thus a co-ordinate covalent bond is formed between N and H+.
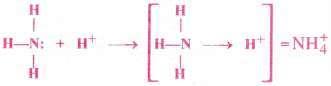
Question 19.
Which hybrid orbitals are used by Carbon atoms in the following molecules?
(a) CH3-CH3 (b) CH3-CH=CH2 (c) CH3-CH2-OH (d) CH3-CHO
Answer:
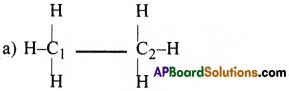
In CH3-CH3, both C1 and C2 atoms are involved in sp³ hybridisation.
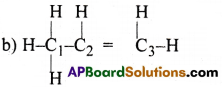
In CH3-CH=CH2, the C1 atom is involved in sp³ hybridisation. And C2 and C3 atoms are involved in sp² hybridisation.
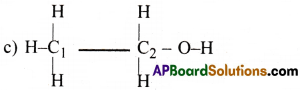
In CH3-CH2OH, both C2 and C2 atoms are involved in sp³ hybridisation.
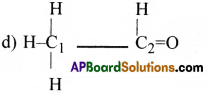
In CH3-CHO, C1 atom is involved in sp³ hybridisation and C2 atom is involved in sp² hybridisation.
Question 20.
What is Hydrogen bond? Explain the different types of Hydrogen bonds with examples. [AP 15, 16, 17, 18] [TS 16, 18, 19, 22]
Answer:
The weak electrostatic force of attraction between Hydrogen atom of one molecule and most electronegative atom of another molecule (or) same molecule is called Hydrogen bond.
Conditions for the formation of Hydrogen bond :
- The size of electronegative atom should be small.
- The electronegativity of the atom to which Hydrogen is attached should be high.
Strength of Hydrogen bond :
The strength of hydrogen bond is between 5-1 OK Cal/mol. It is, thus, weaker than a covalent bond and stronger than Vander Waals forces of attraction.
Types of Hydrogen bonds :
- Inter molecular Hydrogen bond
- Intra molecular Hydrogen bond.
1) Inter molecular Hydrogen bond :
Hydrogen bond formed between two different molecules is called inter molecular hydrogen bond.
Ex : The bonds present in H2O, HF, NH3
![]()
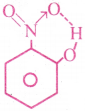
2) Intra molecular hydrogen bond :
A hydrogen bond formed within the same molecule is called intra molecular Hydrogen bond.
Ex : The bonds present in Orthonitrophenol, Ortho hydroxy benzaldehyde.
Question 21.
Explain the formation of H2 molecule on the basis of Valence Bond theory.
Answer:
Valence Bond Theory (VBT) :
This theory explains the shapes of covalent molecules as well as the directions of the bonds in them.
Formation of H2 molecule:
Hydrogen atom contains one unpaired s-orbital. The unpaired s-orbital of one Hydrogen atom directly overlaps axially with another unpaired s- orbital of the other Hydrogen atom and forms one σ bond.

Question 22.
Using Molecular Orbital Theory explain why the B2 molecule is paramagnetic?
Answer:
E.C of Boron atom is 1s²2s²2p¹.
There are 10 electrons in B2 molecule.
In terms of molecular orbital theory’, the electronic configuration of B2 molecule:
(σ 1s)² (σ* 1s)² (σ 2s)² (σ* 2S)2 (π2p¹x = π 2p¹y)
B2 molecule is paramagnetic due to the presence of two unpaired electrons in π2px and π2py orbitals.
![]()
Question 23.
Write the important conditions necessary for linear, combination of atomic orbitals.
Answer:
Linear combination of atomic orbitals to form molecular orbitals takes place under the following conditions:
1) The combining atomic orbitals must have the same or almost equal energy.
Ex: 1s – 1s overlap is possible, but 1s – 2s, 2s – 2p overlaps are not possible.
2) The combining atomic orbitals must overlap to the maximum extent.
Explanation :
Greater the extent of overlap, the greater will be the electron -density between the nuclei of a molecular orbital.
3) The combining atomic orbitals must have the same symmetry about the molecular axis.
Explanation :
By convention, Z-axis is taken as the molecular axis.Even if two atomic orbitals have equal energy, they may not overlap when they do not have the same symmetry.
Allowed overlaps:
s-s, s-pz, px-px, py-py and pz-pz.
Non- allowed overlaps:
s-py, s-px, px-py and px-pz
Question 24.
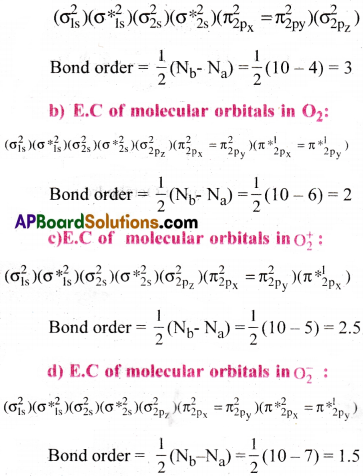
What is meant by term bond order? Calculate the bond orders in the following
a) N2 b) O2 c) O+2 d) O–2 [IPE’ 14][TS 15]
Answer:
Bond Order :
The number of bonds between two atoms of a molecule is called Bond order.
Bond Order (B.O) = \(\frac{1}{2}\) (Nb – Na)
Nb = No.of electrons in bonding orbitals.
Na = No.of electrons in anti bonding orbitals.
a) E.C of molecular orbitals in N2 :
Question 25.
Of BF3 and NF3, dipole moment is observed for NF3 and not for BF3. Why?
Answer:
1) NF3 is a polar molecule with polar bonds. NF3 has unsymmetrical, three dimensional pyramidal structure, with a lone pair of electrons. So the net dipole moment of NF3 is always positive.
2) BF3 is a non-polar molecule with polar bonds. [AP 17]
BF3 has symmetrical and trigonal planar structure, with no lone pair of electrons. So the net dipole moment of BF3 is zero.

Question 26.
Eventhnugh both NH3 and NF3 are pyramidal, NH3 has a higher dipole moment compared to NF3.Whv? [AP-16]
Answer:
In NH3, the direction of the orbital dipole moment due to lone pair is in the same direction of the ‘resultant dipole moment’ of the 3 N-H bonds. Flence, the net dipole moment of NH3 is positive.
But in NF3, molecule the direction of the orbital dipole moment due to lone pair is in the opposite direction to the ‘resultant dipole moment’ of the 3 N-F bonds. Hence, its net dipole moment decreases.
Therefore, dipole moment of NH3 is greater than that of NF3

![]()
Question 27.
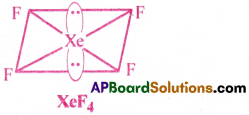
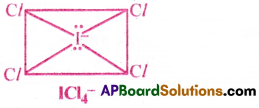
How do you predict the shapes of the following molecules making use of VSEPR theory? (a)XeF4 (b) BrF5 (c) ClF3 and (d) lCl–4 [AP 15]
Answer:
a) In XeF4, the central atom Xe is surrounded by 6 electron pairs.
There are 4 bond pairs and 2 lone pairs. Hence, according to VSEPR theory, to minimise the repulsions between electron pairs, the two lone pairs occupy the opposite comers of Octahedron.
Hence, XeF4 takes the shape of Square planar.
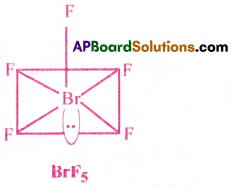
b) In BrF5, the central atom Br is surrounded by 6 electron pairs.
There are 5 bond pairs and 1 lone pair. These six electron pairs are arranged octahedrally around the Br atom.
The lone pair occupy one comer of Octahedron.
Hence, BrF5 takes the shape of square Pyramid.
c) In ClF3, the central atom Cl is surrounded by 5 electron pairs.
There are 3 bond pairs and 2 lone pairs. To minimise the repulsions, the two lone pairs occupy Equatorial positions in Trigonal bipyramid structure.
Hence, ClF3 takes the T-shape.
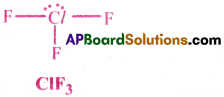
d) In ICl–4, the central atom I is surrounded by 6 electron pairs.
There are 4 bond pairs and 2 lone pairs. Hence, according to VSEPR theory, to minimise the repulsions between electron pairs, the two lone pair occupy the opposite comers of Octahedron.
Hence, ICl–4 takes the shape of square Planar.
Long Answer Questions
Question 1.
Explain the formation of ionic bond with a suitable example. [TS 20]
Answer:
An ionic bond is formed by the complete transfer of electrons from the outer most shell of one atom (with low I.P) to outer most shell of another atom (with high E.A). In this process, each atom attains the nearest noble gas configuration or octet configuration.
The atom which looses electrons becomes cation and the atom which gains electrons becomes anion.The attractive force established between those oppositely charged ions is called ionic bond.
Explanation using the formation ionic bond in NaCl.
The electronic configuration of Na is 1s²2s²2p63s¹
The electronic configuration of Cl is 1s²2s²2p63s²3p5
Na atom is electropositive and has low I.P, and Cl is electronegative and has high E.A.
So, Na atom looses one electron and transfers it to Cl atom. Then Na atom becomes Na+ cation and Cl atom becomes Cl– anion.
Now the electronic configuration of the cation Na* becomes 1s²2s²2p6. = Ne configuration.
The electronic configuration of the anion Cl– becomes 1s²2s²2p63s²3p6 = Ar configuration. Hence, both the ions get the octet configuration.
Thus Na+and Cl– are held together by strong electrostatic forces of attraction called ionic bond.
Question 2.
Explain the factors favourable for the formation of Ionic compounds. [AP 18]
Answer:
Factors which favour the ionic bond formation :
I) Favourable conditions for cation formation:
i) Low ionisation potential :
An atom with low I.P readily looses electron. Therefore it forms cation easily.
ii) Large atomic size :
As the size of the atom increases attraction of the nucleus on valency electrons decreases. Hence large atoms form cations easily compared to small atoms.
iii) Low charge on the ion :
As the charge on the ion increases, the number of electrons to be removed also increases. So, ion w ith low charge is produced more easily.
II) Favourable conditions for anion formation: .
i) High E.A and high E.N :
E.A & E.N measure the tendency of an atom to accept electrons. Therefore atoms with high E.A and E.N readily form anion. Thus halogens form anions readily as they have high E.A and high E.N values.
ii) Small atomic size :
Small atoms hold the electrons gained by them strongly and hence form anions easily.
iii) Low charge on the ion :
The stability of an atom decreases as the negative charge on the ion increases. Therefore anion with low negative charge is formed readily than anions with high negative charge.
Question 3.
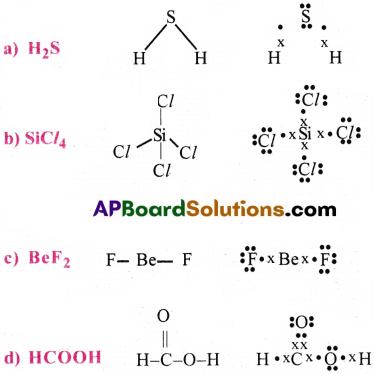
Draw Lewis structures for the following molecules.
(a) H2S (b) SiCl4 (c) BeF2 (d) HCOOH
Answer:
Lewis Structures:
Question 4.
Write notes on a) Bond angle b) Bond Enthalpy c) Bond Length d) Bond order.
Answer:
(a) Bond angle :
The average angle between the bonded orbitals is known as bond angle. It gives some idea about distribution of orbitals around the central atom, in a molecule. It also helps in determining its shape.
Units: degrees.
Ex: H-O-H bond angle in H20 is 104.5°
(b) Bond Enthalpy :
The amount of energy required to break one mole of bonds of a particular type between two atoms in a gaseous state is called bond enthalpy.
Greater the bond enthalpy, stronger is the bond.
Units: kJ/Mol.
Ex: The H-H bond enthalpy in H2 molecule is 435.8 kJ / Mol
(c) Bond Length :
The equilibrium distance between the nuclei of two bonded atoms in a molecule is called bond length. Units: Å
As bond length increases bond enthalpy decreases.
As the number of bonds between two atoms increases, the bond length decreases.
Ex: Bond length of (i) C-C is 1.54 Å, (ii) C=C is 1.33 Å (iii) C≡C is 1.20 Å
(d) Bond Order :
The number of bonds betw een two atoms in a molecule is called bond order. As the bond order increases, the bond length decreases. [AP 19]
Ex: Bond order in H2 is 1 Bond order in N2 is 3
Bond order in O2 is 2 Bond order in O+2 is 2.5
Question 5.
Give an account of VSEPR Theory and its applications.
Answer:
VSEPR Theory means Valence Shell Electron Pair Repulsion Theory.
This theory was proposed to explain the deviations in the bond angles of some molecules.
Postulates of VSEPR theory:
1) The shape of a molecule is determined by the repulsions between all the electron pairs present in the valence shell of central atom.
2) A lone pair of electrons occupies more space around the central atom than bond pair. Because lone pair is attracted to only one nucleus, but the bond pair is attracted by two nuclei.
Hence repulsion between lone pairs is greater than bond pairs.
3) If the central atom contains only bond pairs then the shape of the molecule and bond angles will be according to the expected values.
4) If the central atom contains lone pairs, along with the bond pairs, then the shape of the molecule gets deviated from the expected values. This is due to repulsive forces between various electron pairs. The order of repulsion between various electron pairs :
(lone pair – lone pair) > (lone pair – bond pair) > (bond pair – bond pair)
5) The order of repulsion between various bonds : Triple bond > Double bond > Single bond
Ex: (i) In BeCl2 molecule there are two Be-Cl bonds.
The two bond pairs are arranged in the opposite directions.
The repulsive force between two bond pairs is negligible.
∴ The bond angle is 180°.
ii) In H2O molecule, ‘O’ has two lone pairs. Here the expected bond angle is 109°28’.
But it is reduced to 104°30′ due to the repulsion between two LP-LP and LP-BP.
iii) In CH4 molecule, all the four electron pairs are ‘bond pairs’ only.
Hence the shape of the molecule is Tetrahedral with bond angle 109°28′.
Question 6.
How do you explain the geometry of the molecules on the basis of Valence bond theory?
Answer:
Valence Bond Theory (VBT) :
This theory explains the shapes of covalent molecules as well as the directions of the bonds in them.
Postulates of VBT :
- A covalent bond is formed by the overlapping of half filled atomic orbital of one atom with half filled atomic orbital of another atom.
- The electrons involved in overlapping must have opposite spin.
- Except the bond pair of electrons, the remaining electrons do not loose their identity..
Such electrons are called non-bonding electrons or lone-pair electrons. - Greater the extent of overlap, greater is the strength of covalent bond.
- The direction of covalent bond lies in the direction of maximum overlapping side.
- All atomic orbitals,except s-orbital, are directional. So the bonds formed due to their overlap are also directional.This determines the shape of the molecule.
- A covalent bond formed by the axial overlap of atomic orbitals is called a sigma bond.
Thus head-on overlappings of s-s, s-p , p – p orbitals leads to the formation of σ bonds. - A covalentbond formed by the lateral or sidewise overlap of atomic orbitals is called a π bond.
- A sigma bond is always stronger than a π bond.
Reason: During the formation of a sigma bond, the orbitals overlap along the inter nuclear axis hence the shared pair of electrons is concentrated just in between the nuclei. Where as in ‘π bond formation’ the orbitals overlap laterally. The electron cloud is present, above and below the inter nuclear axis. Hence a sigma bond is always stronger than a ‘π bond’. - Formation of a ‘π bond’ is possible only after the formation of a ‘σ bond’.
- In the case of a double bond, there will be one σ bond and one π bond.
In the case of a triple bond, there will be one σ bond and two π bonds.
Examples :
I) Formation ol H2 molecule :
Each hydrogen atom has one electron in Is orbital. The Is orbitals of 2 hydrogen atoms overlap axially to form a sigma bond between two H atoms.

2) Formation of F2 molecule :
Fluorine has one half filled 2pz orbital. The 2pz orbitals of two fluorine atoms overlap axially to form a sigma bond between two F atoms.

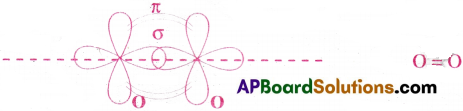
3) Formation of O2 molecule :
The electronic configuration of oxygen is 1s²2s²2p²x2p¹y2p¹z. Oxygen has two half filled p orbitals.The 2py orbital of one atom gverlap axially with 2py orbital of another O atom to form a sigma bond. The 2pz orbitals of tw o oxygen atoms overlap laterally to form a ‘π bond’. Thus a double bond with one strong σ bond and one weak π bond, is formed.
Question 7.
What do you understand by hybridisation? Explain different types of hybridisation involving s and p orbitals. [TS 15, 17, 22]
Answer:
Hybridisation :
The intermixing of atomic orbitals to form new hybrid orbitals is known as hybridisation. The number of hybrid orbitals formed is equal to number of atomic orbitals mixed. There are 3 types of hybridisations involving s and p orbitals. They are sp, sp², sp³ hybridisations.
1) sp hybridisation :
The inter mixing of one ‘s -orbital’ and one ‘p-orbital’ of the outer most shell of an atom is called sp hybridisation.
In this process, we get two ‘sp hybrid orbitals’.The bond angle is 180° and its shape is linear.
Ex : BeCl2, CO2, C2H2.
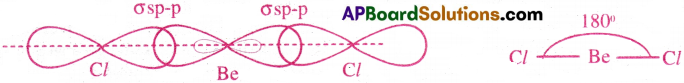
Formation of BeCl2 :
- In BeCl2, the central atom is berylium (Be).
- The electronic configuration of Be (4) in the ground state is 1s² 2s².
- The electronic configuration of Be in the exited state is 1s² 2s¹2px¹2py0 2pz0
- In the excited state, the central Be atom undergoes ‘sp’ hybridisation and forms two ‘sp’ hybrid orbitals.
- The two ‘sp’-orbitals of Be overlap with p-orbitals of two Cl atoms and they form two σ bonds.
- The bond angle is 180° and shape of the BeCl2 is Linear.
2) sp² hybridisation :
The inter mixing of one s-orbital and two p-orbitals of the outer most shell of an atom is called sp² hybridisation.
In this process, we get three sp² hybrid orbitals.The bond angle is 120° and shape is Trigonal planar.
Ex: BCl3, BF3, C2H4.
Formation of BCl3 :
- In BCl3, the central atom is Boron (B).
- The electronic configuration of B(5) in the ground state is 1s² 2s² 2p¹.
- The electronic configuration of B in the excited state is 1s² 2s¹2px¹2py¹2pz0
- In the excited state, the central B atom undergoes sp² hybridisation and forms three sp²hybrid orbitals, each having single electron.
- The three ‘sp²’ orbitals of B overlap with half filled pz orbitals of three Cl atoms in a head – on position and they form three σ bonds.
- The bond angle is 120° and shape of the BCl3 molecule is trigonal planar.

sp³ hybridisation :
The inter mixing of one s-orbital and three p-orbitals of the outer most shell of an atom is called sp³ hybridisation.
Here, we get four sp³ hybrid orbitals. The bond angle is 109° 28′ and shape is Tetrahedral. Ex: CH4, H2O.
Formation of CH4 :
- In CH4, the central atom is Carbon.
- The electronic configuration of C(6) in the ground state is 1s² 2s² 2p².
- The electronic configuration of C in the excited state is 1s² 2s¹2p¹x2p¹y2p¹z
- In the excited state, the central C atom undergoes sp³ hybridisation and forms four sp³ hybrid orbitals, each having single electron.
- The four sp³orbitals of C overlap with half filled s -orbitals of four H atoms in a head – on position and they form four a bonds.
- The bond angle is 109° 28′ and shape of the CH4 molecule is Tetrahedral.

Question 8.
Write the salient features of Molecular Orbital Theory.
Answer:
Molecular Orbital Theory was proposed by Hund and Mulliken. It is explained by LCAO method.
Salient Features of MOT:
- The molecular orbitals are formed when the atomic orbitals of nearly equal energies combined linearly.
- Only such atomic orbitals which are of symmetry with respect to the inter nuclear axis combine to form molecular orbitals.
- The total number of molecular orbitals formed is equal to the total number of combining atomic orbitals. When two atomic orbitals combine, two molecular orbitals are formed. One is called bonding molecular orbital while the other is called anti-bonding molecular orbital.
- Molecular orbitals having lesser energy than atomic orbitals are called bonding molecular orbitals and they are represented by σ and π..
- Molecular orbitals having higher energy than atomic orbitals are called anti- bonding molecular orbitals and they are represented by σ* and π*.
- The order of energies of bonding, anti-bonding and non-bonding orbitals:
Bonding orbitals<Non-bonding<Anti-bonding orbirals. - A molecular orbital is polycentric whereas an atomic orbital is monocentric.
- The shapes of the molecular orbitals depend on the shapes of atomic orbitals.
- Filling up of electrons in the molecular orbitals is done according to Hund’s rule, Paulis exclusion principle and Aufbau principle.
- MOT successfully explained the magnetic nature of molecules.
- MOT is useful to calculate the bond order(number of bonds between atoms) of molecules.
![]()
Question 9.
Give the Molecular Orbital Energy diagram of (a) N2 (b) O2. Calculate the respective bond order. Write the magnetic nature of N2 and O2 molecules. [TS 19]
Answer:
(a) Electronic configuration ofN (7) = 1s² 2s² 2p¹x2p¹y2p¹z.
N atom has 7 electrons. So the molecular orbital of N2 contains 14 electrons.
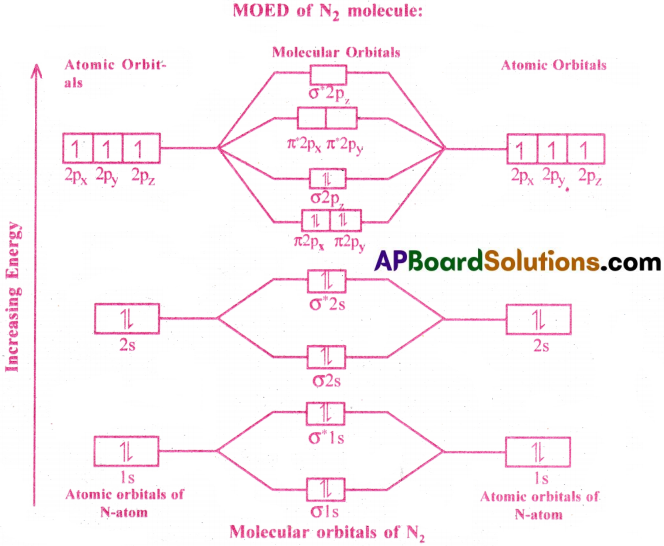
Electronic Configuration of molecular orbitals of N2 :
(σ 1s²)(σ* 1s²)(σ 2s²)(σ* 2s²)(π2p²x = π2p²y)(σ2p²z)
Here, the number of bonding electrons (Nb) = 10
the number of anti-bonding electrons (Na) = 4
Bond order = \(\frac{N_b-N_a}{2}=\frac{10-4}{2}=\frac{6}{2}\) = 3
N2 is diamagnetic due to the absence of unpaired electrons.
(b) Electronic Configuration of O (8) = 1s² 2s² 2p²x2p¹y2p¹z.
Since the O atom has 8 electrons, the molecular orbital of O2 contains 16 electrons.
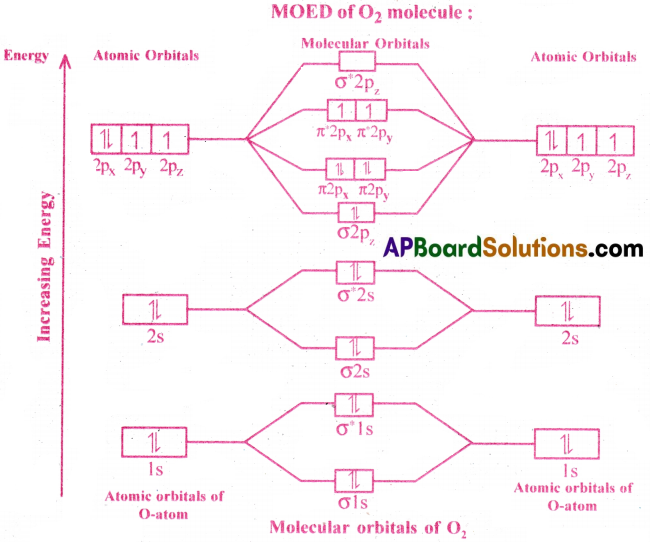
Electronic Configuration of molecular orbitals of O2 :
σ(1s²)σ* (1s²)σ(2s²)σ*(2s²)σ(2p²z)(π2p²x = π2p²y)(π* 2p¹x – π*2p¹y)
Here, the number of bonding electrons (Nb) = 10
the number of anti-bonding electrons (Na) = 6
Bond order = \(\frac{N_b-N_a}{2}=\frac{10-6}{2}=\frac{4}{2}\) = 2
O2 is Paramagnetic due to the presence of two unpaired electrons.
Multiple Choice Questions
Question 1.
The species having bond angles of 120° is
1) ClF3
2) NCl3
3) BCl3
4) PH3
Answer:
3) BCl3
Question 2.
Among the given species identify the isostructural pairs.
1) [NF3 and BF3]
2) [BF–4 and NH+4]
3) [BCl3 and BrCl3]
4) [NH3 & NO–3]
Answer:
2) [BF–4 and NH+4]
Question 3.
The types of hybrid orbitals of nitrogen in NO2+, NO3- and NH4+ respectively are expected to be
1) sp, sp³ and sp²
2) sp, sp² and sp³
3) sp², sp and sp³
4) sp², sp³ and sp
Answer:
2) sp, sp² and sp³
Question 4.
The electronic configuration of the outer most shell of the most electronegative elementis
1) 2s²2p5
2) 3s²3p5
3) 4s²4p5
4) 5s²5p5
Answer:
1) 2s²2p5
![]()
Question 5.
If the electronic configuration of an element is Is2 2s2 2p6 3s2 3p6 3d2 4s2, the four electrons involved in .chemical bond formation will be
1) 3p6
2) 3p6,4s²
3) 3p6, 3d²
4) 3d², 4s²
Answer:
4) 3d², 4s²
Question 6.
In potion the formal charge on the oxygen atom of P-O bond is
1) +1
2) -1
3) -0.75
4) +0.75
Answer:
2) -1
Question 7.
Which of the following angle corresponds to sp2 hybridisation?
1) 90°
2) 120°
3) 180°
4) 109°
Answer:
2) 120°
Question 8.
In NO–3 ion, the number of bond pairs and lone pairs of electrons on nitrogen atom are
1) 2, 2
2) 3, 1
3) 1, 3
4) 4, 0
Answer:
4) 4, 0
Question 9.
Number of π bonds and σ bonds in the adjacent structure is
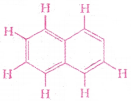
1) 6, 19
2) 4, 20
3) 5, 19
4) 5, 20
Answer:
3) 5, 19
Question 10.
Which of the following species has tetrahedral geometry?
1) BH–4
2) NH–2
3) CO2-3
4) H3O+
Answer:
1) BH–4
Question 11.
In which of the following molecule/ion all the bonds are not equal?
1) XeF4
2) BF–4
3) C2H4
4) SiF4
Answer:
3) C2H4
![]()
Question 12.
In the structure ClF3, the number of lone pairs of electrons on central atom ‘Cl’ is
1) one
2) two
3) four
4) three
Answer:
2) two
Question 13.
Which of the following has the highest dipole moment?
1) CO2
2) HI
3) H2O
4) SO2
Answer:
3) H2O
Question 14.
Match List-1 with List-II
| List I | List II |
| (A) PCl5 | (i) Square Pyramidal |
| (B) SF6 | (ii) Trigonal Planar |
| (C) BrF5 | (iii) Octahedral |
| (D) BF3 | (iv) Trigonal bipyramidal |
Choose the correct answer from the options given below.
1) A-iv, B-iii, C-iii, D-i
2) A-iv, B-iii, C-i, D-ii
3) A-ii, B-iii, C-iv, D-i
4) A-iii, B-i, C-iv, D-ii
Answer:
2) A-iv, B-iii, C-i, D-ii
Question 15.
Which of the following molecules is non-polar in nature?
1) NO2
2) POCl3
3) CH2O
4) SbCl5
Answer:
4) SbCl5
Question 16.
Which of the following pairs of compounds is isoelectronic and isostructural?
1) TeI2, XeF2
2) IBr2, XeF2
3) IF3, XeF2
4) BeCl2, XeF2
Answer:
2) IBr2, XeF2
Quesstion 17.
Which of the following order of energies of molecular orbitals of N2 is correct?
1) (π2py) < (σ2pz) < (π*2px) ≈ (π*2py)
2) (π2py) > (σ2pz) > (π*2px) ≈ (π*2py)
3) (π2py) < (σ2pz) > (π*2px) ≈ (π*2py)
4) (π2py) > (σ2pz) < (π*2px) ≈ (π*2py)
Answer:
1) (π2py) < (σ2pz) < (π2px) ≈ (π*2py)
Question 18.
Consider the following species:
CN+, CN–, NO and CN. Which one of these will have the highest bond order?
1) NO
2) CN–
3) CN+
4) CN
Answer:
2) CN–
Question 19.
Which of the following options represents the correct bond order :
1) O–2 > O2 > O+2
2) O–2 < O2 < O+2
3) O–2 > O2 < O+2
4) O–2 < O2 > O+2
Answer:
2) O–2 < O2 < O+2
![]()
Question 20.
Which one of the following pairs of species have the same bond order?
1) O2, NO+
2) CN–,CO
3) N2O2
4) CO, NO
Answer:
2) CN–,CO
Question 21.
Identify a molecule which does not exist
1) He2
2) Li2
3) C2
4) O2
Answer:
1) He2
Question 22.
Which of the following diatomic molecular species has only n bonds according to Molecular Orbital Theory?
1) Be2
2) O2
3) N2
4) C2
Answer:
4) C2
Question 23.
Which molecule/ion out of the following does not contain unpaired electrons?
1) N+2
2) O2
3) O2-2
4) B2
Answer:
4) B2
Question 24.
Hydrogen bonds are formed in many compounds e.g., H2O, HF, NH3. The correct decreasing order of the boiling points of above compounds is
1) HF > H2O > NH3
2) H2O > HF > NH3
3) NH3 > HF > H2O
4) NH3 > H2O > HF
Answer:
2) H2O > HF > NH3
![]()
Question 25.
In which of the following substances will hydrogen bond be strongest?
1) HCl
2) H2O
3) HI
4) H2S
Answer:
2) H2O