Solving AP 10th Class Physical Science Model Papers and AP 10th Class Physical Science Question Paper June 2022 regularly is an effective strategy for time management during exams.
AP SSC Physical Science Question Paper June 2022 with Solutions
Time: 2 Hours
Maximum Marks: 50
Instructions:
- The question paper consists of 4 sections and 17 questions.
- Internal choice is available only for Q.No.12 in section III and for all the questions in section IV.
- In 2 hours, 15 minutes is allotted to read the question paper.
- All answers shall be written in the answer booklet only.
- Answers shall be written neatly and legibly.
Section-I
(8 × 1 = 8 Marks)
Note:
- Answer ALL the questions.
- Each question carries 1 mark.
Question 1.
What is the S.I. Unit of temperature?
Answer:
Kelvin
Question 2.
Which lens is used to rectify Hypermetropia?
Answer:
Bi-Convex
Question 3.
Write the Lewis dot structure of ‘Helium’.
Answer:
He
![]()
Question 4.
Write the lens formula.
Answer:
\(\frac{1}{\mathrm{f}}=\frac{1}{\mathrm{v}}-\frac{1}{\mathrm{u}}\)
Question 5.
Write any two uses of metals in your daily life.
Answer:
Metals are used in
(i) making conducting wires
(ii) making utensils, making jewelry, etc.
Question 6.
Choose the correct matching:
| Molecule | Shape |
| (a) BeCl2 | (x) Trigonal Planar |
| (b) BF3 | (y) Linear |
| (z) ‘V’ Shape |
(A) a – x, b – y
(B) a – y, b – x
(C) a – x, b – z
(D) a – y, b – z
Answer:
(B) a – y, b – x
Question 7.
Which orbital does the adjacent figure represent?
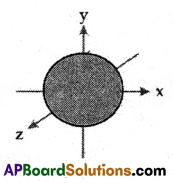
Answer:
s-orbital
Question 8.
Draw the V-I graph of the Ohmic conductor.
Answer:
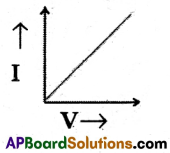
Section-II
(3 × 2 = 6 Marks)
Note:
- Answer ALL the questions.
- Each question carries 2 marks.
Question 9.
Write the materials required to prove Snell’s law.
Answer:
Required material to prove Snell’s law: Pro circle, scale, small black painted plank, semi-circular glass disc, pencil, and laser light.
![]()
Question 10.
For a better understanding of the electronic configuration in an atom, the teacher wrote shorthand notation nlx on the blackboard. Looking at this notation, what could be the probable questions that were generated in the student’s mind? Write any two of them.
Answer:
- What do n, l, x indicate related to atoms?
- How do n, l, x indicate the position of the electrons in the atom?
Question 11.
Kumar told to his teacher that he is not able to recognize the difference between evaporation and boiling. Then the teacher asked some questions and made him understand the difference between them. What are the questions asked by the teacher to Kumar?
Answer:
- Which process takes place rapidly to change from a liquid to a gaseous state?
- In which process temperature remains constant?
- Which process causes cooling?
- Which process is a surface phenomenon?
Section-III
(3 × 4 = 12 Marks)
Note:
- Answer ALL the questions.
- Each question carries 4 marks.
Question 12.
Draw any one of the following diagrams.
(A) Draw a neat diagram of different types of lenses.
(B) Draw a neat diagram of the formation of the ethane molecule with the help of the theory of hybridization.
Answer:
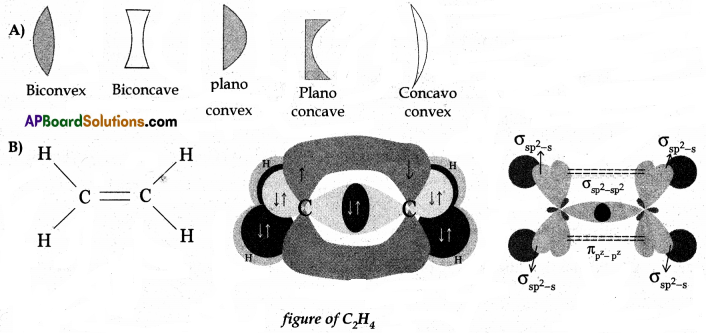
Question 13.
Complete and write the given table.
| Solution | Blue Litmus | Red Litmus | Methyl Orange |
| HCl | ___________ | No Change | ___________ |
| H2SO4 | Red | ___________ | ___________ |
| NaOH | No Change | ___________ | ___________ |
| KOH | ___________ | ___________ | Yellow |
Answer:
| Solution | Blue Litmus | Red Litmus | Methyl Orange |
| HCl | Red | No Change | Red |
| H2SO4 | Red | No Change | Red |
| NaOH | No Change | Blue | Yellow |
| KOH | No Change | Blue | Yellow |
Question 14.
What is the octet rule? How do you appreciate the role of the ‘octet rule’ in explaining the chemical properties of elements?
Answer:
- “The atoms of elements tend to undergo chemical changes that help to leave their atoms with eight outer shell electrons.”
- I appreciate the octet rule. Eight electrons in the outermost shell definitely, give stability to the ion (or) atom.
- Chemically active elements do not have an octet of electrons in the valence shell of their atoms.
- Their reactivity arises from their tendency to achieve the octet, by forming bonds either with atoms of their type or with atoms of other elements.
Section-IV
(3 × 8 = 24 Marks)
Note:
- Answer ALL the questions.
- Each question carries 8 marks.
- Each question has an internal choice.
Question 15.
(A) Define the following:
(a) Calcination
(b) Roasting
(c) Ore
(d) Mineral
(OR)
(B) Explain the formation of a rainbow.
Answer:
(A) (a) Calcination: Calcination is a pyrochemical process in which ore is heated in the absence of air.
(b) Roasting: Roasting is a pyrochemical process in which ore is heated in the presence of air below its melting point.
(c) Ore: The minerals from which the metals are extracted without economical loss are called ore.
(d) Mineral: The elements or compounds of the metals that occur in nature in the earth’s crust are called minerals.
(OR)
(B) Observe the figure.
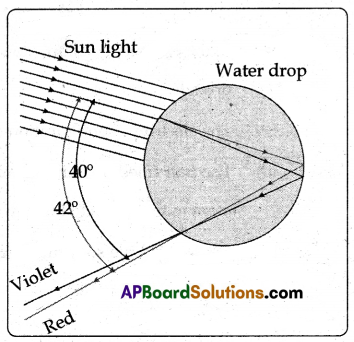
(i) The rays of sunlight enter the drop near its top surface.
(ii) At the first refraction, the white light is dispersed into its spectrum of colours, with violet being deviated the most and red the least.
(iii) Reaching the opposite side of the drop, each colour is reflected into the drop because of total internal reflection.
(iv) Arriving at the surface of the drop, each colour is again refracted into the air.
(v) At the second refraction the angle between red and violet rays further increases when compared to the angle between those at first refraction.
(vi) The angle between the incoming and outgoing rays can be anything between 0° and about 42°.
(vii) We observe a bright rainbow when the angle between incoming and outgoing rays is near the maximum angle of 42°. Diagrammatically it is shown in the figure.
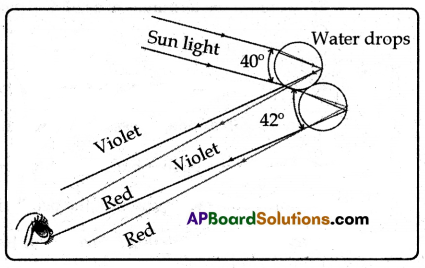
(viii) Although each drop disperses a full spectrum of colours, an observer is in a position to see only a single colour from any one drop depending upon its position.
(ix) If violet light from a single drop reaches the eye of an observer, red light from the same drop can’t reach his eye. It goes elsewhere possibly downwards of the eye of the observer. See the figure.
(x) To see red light, one must look at the drop higher in the sky.
(xi) The colour red will be seen when the angle between a beam of sunlight and light sent back by a drop is 42°.
(xii) The colour violet is seen when the angle between a sunbeam and light sent back by a drop is 40°.
(xiii) If you look at an angle between 40° and 42°, you will observe the remaining colours of VIBGYOR.
![]()
Question 16.
(A) What is hybridization? Explain the formation of the BeCl2 molecule using hybridization.
(OR)
(B) Explain: (A) Brilliance of Diamond; (B) Optical fiber.
Answer:
(A) Hybridisation: The phenomenon of intermixing of orbitals of the same atom which have almost the same energy to form an equal number of new orbitals of equivalent energy is known as hybridization.
Formation of Beryllium Chloride (BeCl2):
(i) The atomic number of Beryllium is 4.
(ii) The electronic configuration of the Beryllium atom in its ground state is 1s22s2.
(iii) The electronic configuration of the Beryllium atom in its excited state is 1s22s12p1.
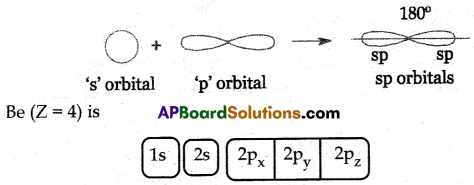
(i) In the excited Beryllium atom its ‘2s’ and ‘2px‘ orbitals intermix to give two equivalent ‘sp’ hybrid orbitals.
(ii) The electronic configuration of Be is 1s22s12px1. It has one half-filled ‘p’ orbital.
(iii) The half-filled 3pz orbitals of two chlorine atoms overlap with ‘sp’ hybrid orbitals of beryllium atom in their axes to form two σsp-p bonds.
(iv) BeCl2 molecule so formed has a linear shape. The bond angle in BeCl2 is 180°.

(OR)
B) (i) Brilliance of Diamond:
- The critical angle of a diamond is very low (24.4°);
- Hence it undergoes total internal reflection of light causing the Brilliance of a diamond.
(ii) Optical Fibre:
- Optical fiber is a very thin fiber made of glass or plastic having a radius of about 1 micrometer.
- Total Internal Reflection of light is the basic principle of working of optical fiber.
Question 17.
(A) Explain Faraday’s law of induction with the help of an activity.
(OR)
(B) Suggest an experiment to prove that the rate of evaporation of a liquid depends on its surface area and vapour already present in the surrounding air.
Answer:
(A) Apparatus: Galvanometer, Coil, Bar magnet.
Procedure:
(i) Connect the terminals of a coil to a sensitive galvanometer as shown in the figure.
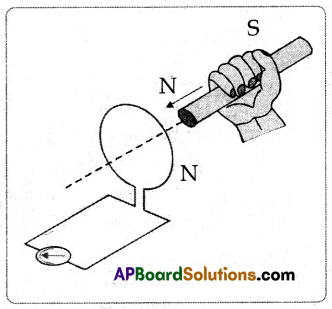
(ii) Normally, we would not expect any deflection of the needle in the galvanometer because there is no EMF in the circuit.
(iii) Now, if we push a bar magnet towards the coil, with its north pole facing the coil, the needle in the galvanometer deflects, showing that a current has been set up in the coil, the galvanometer does not deflect if the magnet is at rest.
(iv) If the magnet is moved away from the coil, the needle in the galvanometer again deflects, but in the opposite direction, which means that a current is set up in the coil in the opposite direction.
(v) If we use the end of the south pole of a magnet instead of the north pole, the result i.e., the deflections in the galvanometer are exactly opposite to the previous one.
(vi) This activity proves that the changes in magnetic flux linked with a closed coil, produce current.
Conclusion:
From this Faraday’s law of induction can be stated as “whenever there is a continuous change of magnetic flux linked with a closed coil, a current is generated in the coil.” This induced emf is equal to the rate of change of magnetic flux passing through it.
(OR)
(B) Rate of evaporation of a liquid depends on its surface area, and the amount of vapour already present in the surrounding air.
Experiment-1:
Aim: To prove the rate of evaporation of a liquid depends on its surface area and vapour already present in the surrounding air.
Apparatus: Two dishes, water
Procedure:
- Take two dishes of different surface areas.
- Pour equal amounts of water into both dishes.
- Observe them after three hours.
- A dish with more surface area has less quantity.
Observation: This shows rate of evaporation increases with increasing surface area.
![]()
Experiment-2:
Aim: To prove the rate of evaporation of a liquid depends on its surface area and vapour already present in the surrounding air.
Apparatus: Two dishes, water
Procedure:
- Take two dishes of equal surface area containing an equal amount of water.
- This observes the dishes one on a more humid day and the second dish on a less humid day.
- We will find that evaporation is less on more humid days due to the presence of more vapour in the air.
Observation: So the rate of evaporation decreases with vapour in the surrounding air.