Solving AP 10th Class Physical Science Model Papers Set 5 regularly is an effective strategy for time management during exams.
AP SSC Physical Science Model Paper Set 5 with Solutions
Time: 2 Hours
Maximum Marks: 50
Instructions:
- The question paper consists of 4 sections and 17 questions.
- Internal choice is available only for Q.No.12 in section III and for all the questions in section IV.
- In 2 hours, 15 minutes is allotted to read the question paper.
- All answers shall be written in the answer booklet only.
- Answers shall be written neatly and legibly.
Section-I
(8 × 1 = 8 Marks)
Note:
- Answer all the questions.
- Each question carries 1 mark.
Question 1.
Convert 30°C into Kelvin Scale.
Answer:
30°C = 273 + 30 K = 303 K
Question 2.
What is the chemical name of common salt?
Answer:
Sodium Chloride
Question 3.
State Pauli’s Exclusion Principle.
Answer:
“No two electrons of an atom have the same four electronic quantum numbers”.
![]()
Question 4.
Statement 1: n + l value of 3s is 3
Statement 2: n + l value of 3s is 4
Write the correct answer to the following in your answer booklet.
(A) Both statements are correct
(B) Statement 1 is only correct
(C) Statement 2 is only correct
(D) Both statements are wrong
Answer:
(B) Statement 1 is only correct
Question 5.
What will happen to the resistance, when the length of a conductor is doubled?
Answer:
Resistance is also doubled as it is directly proportional to length.
Question 6.
Write anyone use of semiconductor.
Answer:
Semiconductors are used to prepare transistors and diodes.
Question 7.
Which of the following has a high velocity of light?
| Substance | Refractive Index |
| Ice | 1.31 |
| Water | 1.33 |
| Benzene | 1.50 |
| Carbon disulphide | 1.63 |
Answer:
Ice
Question 8.
Draw the simple figure of a soap molecule.
Answer:

Section-II
(3 × 2 = 6 Marks)
Note:
- Answer ALL the questions.
- Each question carries 2 marks.
Question 9.
Write any two questions to understand the differences between Myopia and Hypermetropia.
Answer:
- What is myopia?
- What is hypermetropia?
- Where is the image formed in the myopic eye?
- Where is the image formed in the hypermetropic eye?
![]()
Question 10.
Plaster of Paris should be stored in a moisture-proof container. Explain Why?
Answer:
- Plaster of Paris means calcium sulphate hemihydrate (CaSO4 . \(\frac{1}{2}\) H2O)
- Plaster of Paris on mixing with water, it sets into a hard solid mass due to the formation of gypsum (CaSO4 . 2H2O)
CaSO4 . \(\frac{1}{2}\) H2O + 1\(\frac{1}{2}\) H2O → CaSO4 . 2H2O - Because of the above reason Plaster of Paris should be stored in a moisture-proof container.
Question 11.
What happens when a small piece of sodium is dropped into ethanol?
Answer:
When a small piece of sodium is dropped into ethanol it releases Hydrogen gas.
2C2H5OH + 2Na → 2C2H5ONa + H2 ↑
Section-III
(3 × 4 = 12 Marks)
Note:
- Answer ALL the questions.
- Each question carries 4 marks.
Question 12.
Draw any one of the following diagrams:
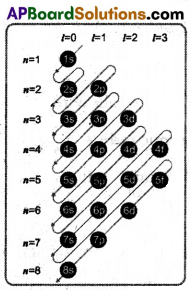
(A) Draw the diagram that shows the increasing value of (n + l).
(B) Draw the ray diagrams to find the images when an object is placed in front of the lens (i) at a distance of 8 cm, and (ii) at a distance of 10 cm on the principal axis of a convex lens whose focal length is 4 cm. Write the characteristics of images in both the cases.
Answer:
(A) Increasing order of orbitals with their relative energies
1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p < 8s
(B) (i) Ray Diagram:
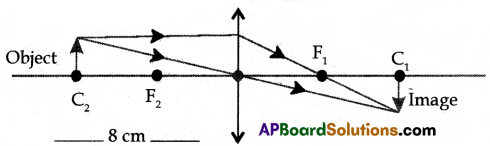
Characteristics of image:
- The size of the image is equal to the size of the object.
- Inverted image.
- Real image
- Image formed at C1
(ii) Ray Diagram:
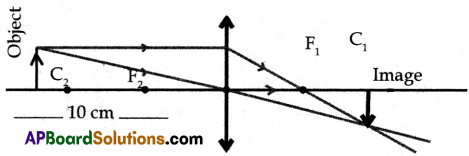
Characteristics of image:
- Image size is less than that of object size.
- Inverted image.
- Real image
- Image is formed in between F1 & C1
![]()
Question 13.

Based on the above pH scale values, answer the following questions.
- Which is the strongest acid among A, B, C, D, E?
- Which is the strongest base among A, B, C, D, E?
- Which is the weakest base among A, B, C, D, E?
- Which is neutral in natural among A, B, C, D, E?
Answer:
- A is the strongest acid
- E is the strongest base
- D is the weakest base
- C is the neutral
Question 14.
What is the reason behind the shining of diamonds and how do you appreciate it?
Answer:

- Total internal reflection is the main cause of the brilliance of diamonds.
- The critical angle of diamonds is shallow (24.4°)
- Due to repeated internal reflections diamond sparkles.
- By cutting the diamond in such a way that the incident angle at each place is greater than the critical angle (C), T.I.R will take place again and again.
- The simple transparent crystal by cutting utilizes the principle of T.I.R and gets the same brilliance as the diamond.
Section-IV
(3 × 8 = 24 Marks)
Note:
- Answer ALL the questions.
- Each question carries 8 marks.
- Each question has an internal choice.
Question 15.
(A) Define the following.
(1) Faraday’s Law
(2) Lenz’s Law
(OR)
(B) What is Octet rule? Explain the role of the Octet rule in explaining the chemical properties of elements.
Answer:
(A) 1. Faraday’s law: The induced EMF generated in a closed loop is equal to the rate of change of magnetic flux passing through it.
2. Lenz’s law: The induced current will appear in such a direction that it opposes the changes in the flux in the coil.
(OR)
(B) Octet Rule: The atoms of the elements tend to get eight electrons in their valence shell by participating in chemical reactions, which is called the Octet rule.
Role of Octet rule in chemical properties of elements:
- All the inert gases have an octet configuration except helium.
- So they do not participate in any chemical reactions.
- If any group of elements tries to get an octet configuration by losing or sharing electrons to get stability, they are said to be following the Octet rule.
- In this way, the Octet rule helps in explaining the chemical properties of elements.
Question 16.
(A) How do the following properties change in a group and period? Explain.
(A) Atomic radius
(B) Ionization energy
(C) Electron affinity
(D) Electronegativity
(OR)
(B) Write about the cleansing action of soap.
Answer:
(A) (A) Atomic radius: The distance between the center of the nucleus to the outermost shell of an atom is called the atomic radius.
In Groups: Atomic radius increases from top to bottom in a group.
In Periods: Atomic radius decreases from left to right in a period.
(B) Ionization energy: The energy required to remove an electron from the outermost orbit of a neutral gaseous atom is called Ionization energy.
In Groups: Ionization energy decreases from top to bottom in a group.
In a Period: Ionization energy generally increases from left to right in a period.
(C) Electron affinity: The electron affinity of an element is defined as the energy liberated when an electron is added to its neutral gaseous atom.
In Groups: Electron affinity decreases as we go down in a group.
In Periods: Electron affinity increases along a period from left to right.
(D) Electronegativity: The electronegativity of an element is defined as the relative tendency of its atom to attract electrons towards it when it is bound to the atoms of another element.
In groups: Electronegativity decreases as we go down in a group.
In periods: Electronegativity increases along a period from left to right.
(OR)
(B) (i) Soap molecule has one polar end and one non-polar end.

(ii) The polar end is hydrophilic and attracted to water.
(iii) The non-polar end is hydrophobic and attracted towards grease or oil on cloth.
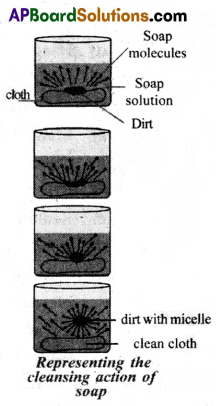
(iv) Suppose that we a put dirty cloth in the soap solution, the list is mainly greasy matter.
(v) When soap dissolves in water, its hydrophobic end attaches itself to dirt and removes it from cloth.
![]()
Question 17.
(A) Explain the experimental procedure of finding the specific heat of a solid.
(OR)
(B) State Ohm Laws and explain with an activity.
Answer:
(A) Aim: To find the specific heat of a given solid.
Apparatus: Calorimeter, thermometer, stirrer, water, steam heater, wooden box and lead shots.
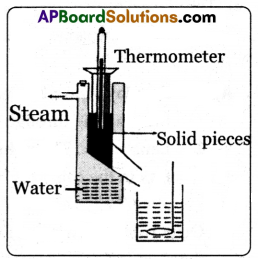
Procedure:
(i) Measure the mass of the calorimeter along with the stirrer.
Mass of calorimeter = m1
(ii) Now fill one-third of the volume of the calorimeter with water. Measure its mass and its temperature.
Mass of calorimeter with water = m2
∴ Mass of water = m2 – m1
Temperature of water in calorimeter = T1
(iii) Take a few lead shots and place them in hot water or steam heater. Heat them upto a temperature 100°C. Let this temperature be T2.
(iv) Transfer the hot lead shots quickly into the calorimeter (with minimum loss of heat.) We will observe that the mixture settles to a certain temperature after some time.
(v) Measure this temperature T3 and mass of calorimeter along with contents (water and lead shots).
Mass of the calorimeter along with contents = m3
Mass of the lead shots = m3 – m2
(vi) Since there is no loss of heat to surroundings, we can assume that the entire heat lost by the solid is transferred to the calorimeter and water to reach the final temperature.
(vii) Let the specific heats of the calorimeter, lead shots, and water be Sc, Sl, and Sw respectively.
According to the method of mixtures, we know
Heat lost by the solid = Heat gain by the calorimeter + Heat gain by the water
(m3 – m2) Sl (T2 – T3) = m1 Sc (T3 – T1) + (m2 – m1) Sw (T3 – T1)
Sl = \(\frac{\left[\mathrm{m}_1 \mathrm{~S}_{\mathrm{c}}\left(\mathrm{T}_3-\mathrm{T}_1\right)+\left(\mathrm{m}_2-\mathrm{m}_1\right) \mathrm{S}_{\mathrm{w}}\left(\mathrm{T}_3-\mathrm{T}_1\right)\right]}{\left(\mathrm{m}_3-\mathrm{m}_2\right)\left(\mathrm{T}_2-\mathrm{T}_3\right)}\)
(viii) Knowing the specific heat of the calorimeter and water, we can calculate the specific heat of the solid (lead shots).
(OR)
(B) Ohm’s Law: The potential difference between the ends of a conductor in a circuit is directly proportional to the flow of current in the circuit.
V ∝ I (or) \(\frac{V}{I}\) = constant
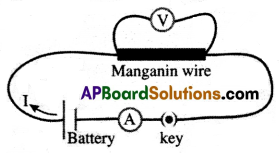
Verification/Experiment:
(i) Set up a circuit as shown in the figure.
(ii) The figure consists of a nichrome wire AB of length, say 0.5m, an ammeter, voltmeter, and four cells of 1.5V each.
(iii) First use only one cell as the source in the circuit.
(iv) Note the reading in the ammeter (I), for the current and reading of the voltmeter (V) for the potential difference across the nichrome wire AB in the circuit.
(v) Now tabulate the values in the table as mentioned below.
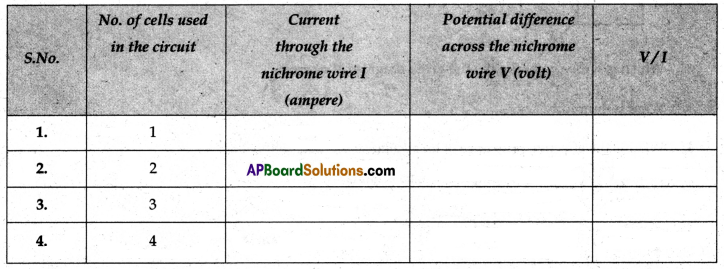
(vi) Next connect two cells in the circuit and note the respective readings of the ammeter and voltmeter for the values of current through the nichrome wire and potential difference across the nichrome wire.
(vii) Repeat the above steps using three cells and then four in the circuit separately.
(viii) Calculate the ratio of V to I for each pair of potential difference V and current I.
(ix) Plot a graph between V and I, and observe the nature of the graph.
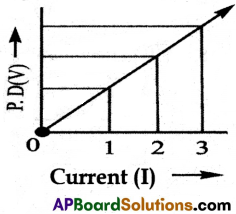
(x) From the above activity we will find that approximately the same value for \(\frac{V}{I}\) is obtained in each case.
(xii) Thus the V-I graph is a straight line that passes through the origin of the graph as shown above.
(xiii) From the above graph \(\frac{V}{I}\) is a constant ratio, hence Ohm’s law is verified.