Solving AP 10th Class Physical Science Model Papers Set 2 regularly is an effective strategy for time management during exams.
AP SSC Physical Science Model Paper Set 2 with Solutions
Time: 2 Hours
Maximum Marks: 50
Instructions:
- The question paper consists of 4 sections and 17 questions.
- Internal choice is available only for Q.No.12 in section III and for all the questions in section IV.
- In 2 hours, 15 minutes is allotted to read the question paper.
- All answers shall be written in the answer booklet only.
- Answers shall be written neatly and legibly.
Section-I
(8 × 1 = 8 Marks)
Note:
- Answer all the questions.
- Each question carries 1 mark.
Question 1.
Identify the wrong statement.
X: Acids react with metals and produce CO2 gas.
Y: Acids react with metals and produce H2 gas.
Answer:
Statement X: Acids react with metals and produce CO2 gas.
Question 2.
What happens to the light ray, when it strikes the interface normally?
Answer:
No deviation
Question 3.
Your grandfather has ‘Presbyopia’. Which lens do you suggest to your grandfather?
Answer:
Bifocal Lens
![]()
Question 4.
I am the element belonging to the Halogen family and I have the highest electro-negativity value. Who am I?
Answer:
Fluorine
Question 5.
What is wrong in the given diagram?

Answer:
Direction of magnetic lines of force. In the given diagram magnetic lines of force are directed from North to South. But they are from South to North.
Question 6.
Which of the following is not an alkane?
CH4, C3H8, C2H4, C5H12
Answer:
C2H4
Question 7.
Convert 20°C into Kelvin scale.
Answer:
Kelvin Scale = Celsius Scale + 273
= 20°C + 273
= 293 K
∴ 20°C = 293 K
Question 8.
Read the information of an electron and answer the following.
| n | 1 | ml | ms |
| 3 | 1 | 3 | +1/2 |
(i) Name the orbital of the electron.
Answer:
3p orbital
Section-II
(3 × 2 = 6 Marks)
Note:
- Answer ALL the questions.
- Each question carries 2 marks.
Question 9.
Prepare some questions to know the differences between evaporation and boiling.
Answer:
- What is the temperature of water (substance) when evaporation takes place?
- What is the boiling point of water (substance)?
- Is the temperature of all the water (substance) the same when evaporation takes place?
- Is the temperature of all the water (substance) the same when boiling takes place?
- What happens to the temperature of the remaining water (substance) when evaporation takes place?
- Does boiling take place at all temperatures?
Question 10.
Which method do you suggest for the extraction of high-reactivity metals? Why?
Answer:
High reactivity metals like K, Ca, Mg, etc., can be extracted by electrolysis.
Reasons:
- Simple reduction methods like heating with C, Co, etc., to reduce the ores of these metals are not feasible.
- The temperature required for the reduction is too high and more expensive.
- Hence electrolysis is the suggestible method to extract highly reactive metals.
![]()
Question 11.
Rajkumar said to you that the magnetic field lines are open and they start at the north pole of a bar magnet and end at the south pole. What questions do you ask Rajkumar to correct him by saying “field lines are closed”?
Answer:
I asked Rajkumar some questions to correct him.
- Are the magnetic field lines, closed or open loops?
- How do the field lines behave inside the magnet?
- Why is the magnetic compass needle following a curved path from one pole to another?
- What do field lines indicate?
- What is the direction of the field line inside the magnet?
- Is the direction of field lines, from its south pole or north pole?
Section-III
(3 x 4 = 12 Marks)
Note:
- Answer ALL the questions.
- Each question carries 4 marks.
Question 12.
Draw any one of the following diagrams:
(A) Draw the ray diagrams to find the images when an object is placed in front of the lens (i) at a distance of 8 cm, and (ii) at a distance of 10 cm on the principal axis of a convex lens whose focal length is 4 cm. Write the characteristics of images in both the cases.
(B) Draw simple diagrams to show how electrons are arranged in the following covalent molecules:
(a) Calcium Oxide (CaO)
(b) Water (H2O)
(c) Chlorine (Cl2)
Answer:
(A) (i) Ray Diagram:
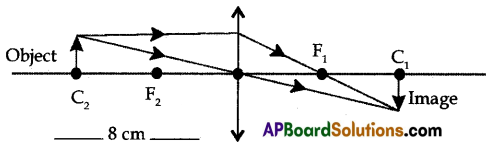
Characteristics of Image:
(a) Size of the image equal to the size of the object.
(b) Inverted image
(c) Real image
(d) Image formed at C1
(ii) Ray Diagram:
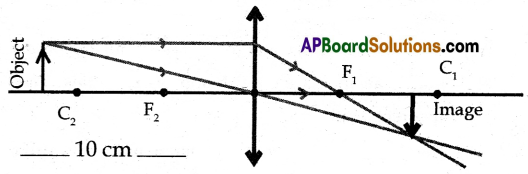
Characteristics of Image:
(a) Image size is less than that of object size.
(b) Inverted image.
(c) Real image
(d) Image is formed in between F1 & C1
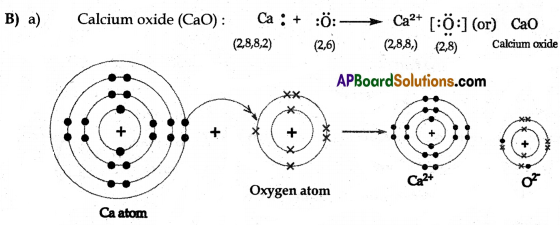
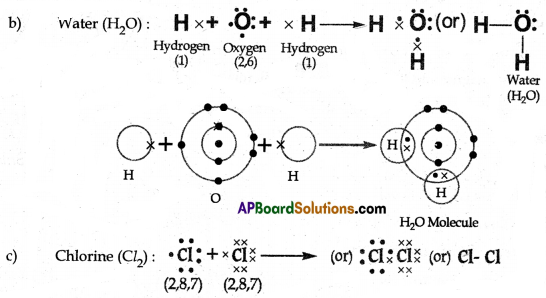
Question 13.
| Organic Compound | Methane | Ethane | Propene | Butene | Pentyne | Hexyne |
| Formula | CH4 | C2H6 | C3H6 | C4H8 | C5H8 | C6H10 |
Observe the above table and answer the following questions.
(i) Write the general formula of Alkanes.
(ii) Mention the names of unsaturated hydrocarbons.
(iii) Write the homologous series of Alkynes.
(iv) Write the general formula of Alkynes.
Answer:
(i) The general formula of alkanes is CnH2n+2
(ii) Unsaturated hydrocarbons from the given table.
C3H6 (Propene), C4H8 (Butene), C5H8 (Pentyne), C6H10 (Hexyne)
(iii) Homologous series of alkynes C2H2, C3H4, C4H6,………
(iv) The general formula of Alkyne is CnH2n-2
![]()
Question 14.
Why is it difficult to shoot a fish swimming in water?
Answer:
- It is due to refraction.
- If the object and observer are situated in different mediums then due to refraction, the object appears to be displaced from its real position.
- The shooter cannot observe the exact position of the fish.
- When the fish is in water (denser medium) and the observer is in the air (rarer medium) due to refraction at the water-air interface the fish appears to be raised and seems to be close to the surface which is called apparent depth.
- The shooter aims the gun at the apparent position of the fish instead of the real position of the fish.
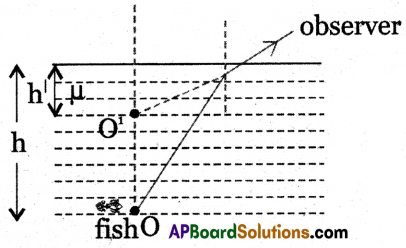
h – real depth
h’ – apparent depth
o – real position of the fish
o’ – apparent position of the fish - So it is difficult to shoot the fish.
Section-IV
(3 × 8 = 24 Marks)
Note:
- Answer ALL the questions.
- Each question carries 8 marks.
- Each question has an internal choice.
Question 15.
(A) Explain the working of an AC electric generator with a neat diagram.
(OR)
(B) (i) Explain why dogs pant during hot summer days using the concept of evaporation.
(ii) Why do we get dew on the surface of a cold soft drink bottle kept in open air?
Answer:
(A) (i) Electric Generator or Dynamo: It is a device that converts mechanical energy into electrical energy.
(ii) Principle: It works on the principle of electromagnetic induction.
(iii) Construction: It consists of an Armature coil, Brushes, Slip rings, a Strong magnet, and a Rotating mechanism (or) motor. The two A and B of the coil ABCD are connected to the slip rings.
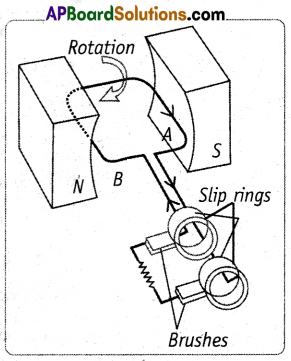
Working:
(i) When the coil is at rest in a vertical position, with side (A) of the coil at the top position and side (B) at the bottom position, no cur¬rent will be induced in it. Thus current in the coil is zero at this position.
(ii) When the coil is rotated in a clockwise direction, current will be induced in it and it flows from A to B, in this position the current increases from zero to a maximum.
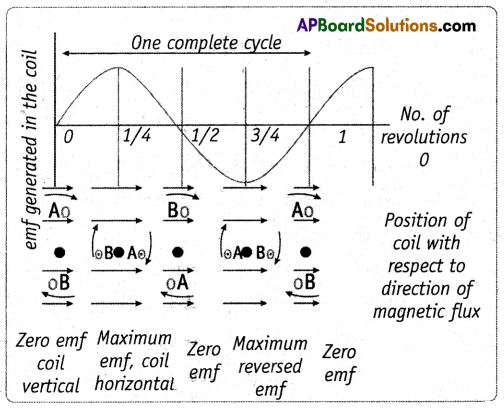
(iii) If we continue the rotation of the coil current decreases during the second quarter of the rotation and once again becomes zero when the coil comes to a vertical position with side B at the top side and side A at the bottom position.
(iv) During the second half of the rotation, the current generated follows the same patterns as that in the first half, except that the direction of current is reversed.
(v) Thus, after every’ rotation of the current in the respective arm changes, thereby generating an alternating current. This device is called an A.C. generator.
(OR)
(B) (i) 1. Dogs pant during hot summer days and get their body cooled. This cooling effect is due to evaporation. Evaporation is a surface phenomenon.
2. Temperature of a system falls during evaporation. During summer the temperature in the human body increases the temperature of the skin. As a result, the water in the sweat glands starts evaporating. Evaporation is a cooling process in which the human body gets cooled.

3. Dogs don’t have sweat glands. Their body is covered with hair. They have sweat glands only in
their feet.
4. So by panting the water on the tongue evaporates resulting in the cooling of the dog’s body.
(ii) 1. It is due to condensation.
2. Condensation is the phase change from gas to liquid.
3. When a cold soft drink bottle is kept in the open air, the water vapour present in the surrounding air condenses on the bottle.

4. The water molecules are slowed down and stick to the surface of the bottle as its surface is cold.
5. These water droplets are seen as dew on the surface of the bottle.
Question 16.
(A) Explain the significance of three quantum numbers in predicting the positions of an electron in an atom.
(OR)
(B) Write the difference between an ionic bond and a covalent bond.
Answer:
(A) Each electron in an atom is described by a set of three quantum numbers n, l, and ml. These numbers indicate the probability of finding the electron in the space around the nucleus.
(a) Principal quantum number (n):
- Niels Bohr introduced it.
- The principal quantum number explains the size and energy of the orbitals.
- These orbitals are called energy levels or shells. It is represented by ‘n’, where n = 1, 2, 3,..etc.
- As ‘n’ increases, the shells become larger and the electrons in those orbitals are farther from the nucleus.
- As ‘n’ increases the energy of the shells also increases.
- The number of electrons in a shell is limited to 2n2.
- The shells are denoted by the letters K, L, M, N,……. etc.
| Shells | K | L | M | N | O |
| n | 1 | 2 | 3 | 4 | 5 |
(b) Orbital Quantum Number (l) (Or) Angular Momentum Quantum Number (l):
- Sommerfeld introduced it.
- This quantum number defines the shape of the orbital occupied by the electron and the orbital angular momentum of the electron in motion.
- Due to this fact, it is also called angular momentum quantum number. It is represented by ‘l’.
- ‘l’ has integer values from 0 to (n – 1) for each value of n.
- Each value of l is related to the shape of orbitals in the space around the nucleus.
| l | 0 | 1 | 2 | 3 |
| Name of the Orbital | s | p | d | f |
- These orbitals (s, p, d, f….) are generally called sub-shells.
- Orbitals have the same value of ‘n’ but different values of ‘l’.
- The quantum number ‘l’ also governs the degree to which the electron is attached to the nucleus.
- The larger the value of ‘l’, the smaller the bond with which it is attached to the nucleus.
(c) Magnetic Orbital Quantum Number (m1):
- To explain the Zeeman effect and Stark effect, ‘magnetic orbital quantum number’ is introduced by Lande.
- The orientation of the orbital with the external magnetic field determines the magnetic orbital quantum number (ml).
- ml has integer values between -l and +l including zero. Thus for a certain value of ‘l’ there are (2l + 1) integer values for ml. They are -l, -l + 1 …… 0, l – 1, l.
- These values describe the orientation of the orbital in space relative to the other orbitals in the atom when it is kept in a strong magnetic field.
- When l = 0, (2l + 1) = 1, there is only one value of ml. When l = 1, (2l + 1) = 3, that means ml has three values namely, -1, 0, +1.
- The orientations of these three are along the x, y, and z axes. These are labelled as px, py, and pz.
- Orbitals in the sub-shell belonging to the same shell possess the same energy with different orientations, these are called degenerate orbitals.
Spin Quantum Number (ms):
- Uhlenbeck and Goldsmith introduced it.
- It is denoted by the letter ‘ms‘.
- This quantum number refers to the two possible orientations of the spin of an electron, one clockwise (↑) and the other anticlockwise (↓) spin.
- The spin motion of the electrons is represented by \(+\frac{1}{2}\) and \(-\frac{1}{2}\).
(OR)
(B)
| Ionic Bond | Covalent Bond |
| 1. An ionic bond is formed by the transfer of one or more electrons from one other. | 1. A covalent bond is formed by the mutual sharing of electrons between atoms and atoms. |
| 2. It is formed between metal and non-metal. | 2. It is formed between non-metals. |
| 3. It is also called an electrovalent bond and is due to electrovalence. | 3. It is called an electron pair bond and is due to covalency. |
| 4. Ionic bond consists of an electrostatic force of attraction between the oppositely charged ions. | 4. A covalent bond consists of a shared pair or pairs of electrons that are attracted by both nuclei. |
| 5. Ionic bonds are non-rigid and non-directional. | 5. Covalent bonds are rigid and directional. |
| 6. Ionic bonds are polar. | 6. Covalent bonds may be polar or non-polar. |
![]()
Question 17.
(A) How do you find experimentally the refractive index of a material of a prism?
(OR)
(B) Write an activity to show that the solutions of compounds like alcohol and glucose do not show acidic character even though they are having Hydrogen.
Answer:
(A) Aim: To find the refractive index of a prism.
Material Required: Prism, piece of white chart of size 20 × 20 cm pencil, pins, scale, and protractor.
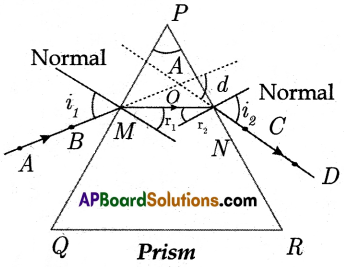
Procedure:
- Let us take a prism and place it on the white chart in such a way that the triangular base of the prism is on the chart.
- Let us draw a line around the prism using a pencil, having vertices P, Q and Rand remove the prism.
- Measure the angle between PQ and QR which gives the angle of prism (A).
- Let us consider a light ray ‘AB’ incident at ‘M’. Draw a ‘Normal’ at ‘M’.
- Let us mark ‘M’ on PQ and draw a perpendicular to PQ at M.
- Let us mark an angle of 30° and draw a line ‘AB’ at ‘M’ which gives the incident ray. The angle of incidence is ‘i’.
- Fix two pins vertically on the line AB.
- Now let us look for the images of two pins through the prism on the other side and fix another two pins say ‘C’ and ‘D’.
- Remove the prism and draw a line to PR which passes through C and D points. This line gives an emerging ray.
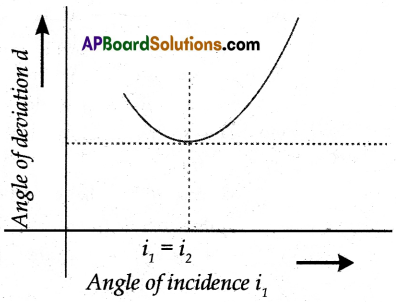
- Draw a Normal to ‘PR’ and measure the angle between CD and Normal, which gives the angle
- Now extend the both incident and emergent ray till they meet at a point ‘O’.
- Measure the angle between the extended two rays which gives an angle of deviation (d). Plot a graph between i and d.
- As the angle of incidence changes angle of deviation also changes.
- As the angle of incidence increases, the angle of deviation decreases and attains a minimum value (Angle of minimum deviation) and further it increases with an increase in angle of incidence.
- Now tabulate the reading of angle of incidence (i1), angle of emergence (i2), and angle of deviation (d).

- The Angle of the prism is A.
- The angle of minimum deviation is D. Then the refractive index of prism ‘n’ = \(\frac{\sin \left(\frac{A+D}{2}\right)}{\sin \frac{A}{2}}\)
(OR)
(B)
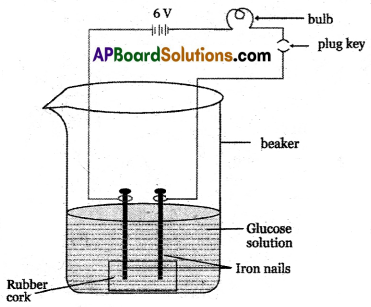
- Prepare solutions of glucose and alcohol.
- Fix two iron nails on a rubber cork and place the cork in a beaker as shown in the figure.
- Connect the nails to the two terminals of a 6-volt DC battery through a switch and a bulb.
- Now pour glucose solution (C6H12O6) and switch on the current.
- The bulb does not glow. This shows that glucose solution does not conduct electricity.
- Repeat this experiment with alcohol solution in the beaker. The bulb does not glow again. That means the alcohol solution does not conduct electricity.
- Due to the absence of ions in glucose and alcohol solutions, they do not conduct electricity.
- Glucose and alcohol do not dissociate in water to produce H+ ions even though they contain hydrogen.
- Glucose and alcohols are not categorized as acids because they do not produce H+ ions in aqueous solution.