Solving AP 10th Class Physical Science Model Papers Set 1 regularly is an effective strategy for time management during exams.
AP SSC Physical Science Model Paper Set 1 with Solutions
Time: 2 Hours
Maximum Marks: 50
Instructions:
- The question paper consists of 4 sections and 17 questions.
- Internal choice is available only for Q.No.12 in section III and for all the questions in section IV.
- In 2 hours, 15 minutes is allotted to read the question paper.
- All answers shall be written in the answer booklet only.
- Answers shall be written neatly and legibly.
Section-I
(8 × 1 = 8 Marks)
Note:
- Answer all the questions.
- Each question carries 1 mark.
Question 1.
Convert 40°C into Kelvin scale.
Answer:
40°C
K = C + 273
= 40 + 273
= 313 K
∴ 40°C = 313 K
Question 2.
Answer the following questions by observing the table given below.
| Material | Refractive Index |
| Water | 1.33 |
| Ice | 1.31 |
(A) Which is a denser medium?
(B) In which medium light travels faster?
Answer:
(A) Water (with a higher refractive index) is a denser medium.
(B) Light travels faster in ice.
![]()
Question 3.
Draw the shape of the ‘s’ orbital.
Answer:

Question 4.
3Ω, 3Ω resistances are connected in series. Calculate the resultant resistance.
Answer:
In series, Rs = R1 + R2
= 3Ω + 3Ω
= 6Ω
Question 5.
List any two metals that are found in nature as oxides.
Answer:
Aluminium, Manganese, Ferrus (Iron), and Zinc are the metals that are found in nature as oxide ores.
Question 6.
Statement 1: Alkanes undergo substitution reactions only.
Statement 2: Alkanes undergo addition reactions only.
Choose the correct option and write it in your answer booklet.
(A) Only statement 1 is correct.
(B) Only statement 2 is correct.
(C) Both 1 and 2 statements are correct.
(D) Both 1 and 2 statements are wrong.
Answer:
(A) Only statement 1 is correct.
Question 7.
How will the image formed by a convex lens be affected if the upper half of the lens is wrapped with black paper?

Answer:
The brightness of the image is reduced.
Question 8.
Who introduced “The Concept of Octaves”?
Answer:
Newlands
Section-II
(3 × 2 = 6 Marks)
Note:
- Answer all the questions.
- Each question carries 2 marks.
Question 9.
Assume and write why the sky is blue.
Answer:
- The sky appears blue due to a phenomenon known as scattering of light, which may be defined as the re-emission of light by atoms or molecules in all directions with different frequencies when compared to incident frequency.
- An atom will respond to incoming light only when the wavelength is comparable to the size of the atom. The majority of Earth’s atmosphere is composed of nitrogen and oxygen molecules.
- The atomic size of these molecules matches the wavelength of violet and blue colour light. So, these colours are scattered more giving beautiful blue colour to the sky.
- Even though violet colour undergoes maximum scattering, we see the sky to be blue because the human eye is more sensitive to blue colour than violet colour light.
![]()
Question 10.
Pose any two questions about the Modern Periodic Table.
Answer:
- What is Modern Periodic Law?
- What is the property of elements that the Modern Periodic Table is based on?
- How many periods and groups are there in Modern Periodic Table?
- Which group of elements of the Modern Periodic Table are called Chalcogens?
Question 11.
Write the bond angles of BeCl2 and BF3.
Answer:
- The bond angle of BeCl2 is 180°.
- The bond angle of BF3 is 120°.
Section-III
(3 × 4 = 12 Marks)
Note:
- Answer all the questions.
- Each question carries 4 marks.
Question 12.
Draw any one of the following diagrams.
(A) Draw a ray diagram for the image formed when an object is kept at 2F2 before a convex lens.
(B) Draw die elliptical orbits for main quantum numbers n = 4 by the Bohr-Sommerfeld model.
Answer:
(A) Object Placed at 2F2:
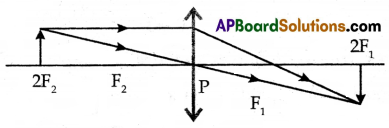
Properties of the Image:
Real: The image is formed by the actual intersection of light rays.
Magnification: The magnification produced is perpendicular, indicating that the size of the image is equal to the size of the object.
(B)
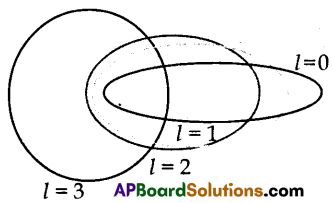
Question 13.
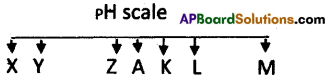
The values from X to M are in ascending order. The value of A is 7. Then:
- Which of the letters represents a strong acid?
- Out of K, L which is a strong base?
- Which of the letters represents a weak base?
- Which letter represents neutral?
Answer:
- X and Y are strong acids.
- L is a stronger base than K.
- K and L are weak bases.
- A is neutral.
![]()
Question 14.
Write any four uses of carbon and its allotropes.
Answer:
- Sugar, Glucose, Proteins, etc; are all made of carbon.
- Carbon is also used for determining the age of fossils in the Carbon dating method.
- Diamond, an allotrope of carbon is used in jewelry.
- Graphite, an allotrope of carbon is used as lead in pencils and as electrodes in batteries, and also used as a lubricant.
- Nanotubes are used as molecular wires. Nanotubes are used to insert bio-molecules into a single cell.
Section-IV
(3 × 8 = 24 Marks)
Note:
- Answer all the questions.
- Each question carries 8 marks.
- Each question has an internal choice.
Question 15.
(A) Compare and contrast between Evaporation and Condensation.
(OR)
(B) Explain the working procedure of the motor.
Answer:
(A)
| Evaporation | Condensation |
| (i) The process of escaping molecules from the surface of a liquid at any temperature is called Evaporation. | (i) Condensation is the process of phase change from gas to liquid. |
| (ii) Evaporation takes place at any temperature. | (ii) Condensation takes place at any temperature. |
| (iii) During evaporation temperature of the system decreases. Hence it is a cooling process. | (iii) During condensation temperature of the surroundings increases. Hence it is a warming process. |
| (iv) Evaporation is a surface phenomenon. | (iv) Condensation is a bulk phenomenon. |
| (v) Drying of wet clothes, and the formation of clouds are examples of evaporation. | (v) Formation of dew and fog are examples of condensation. |
(B) Electric Motor: An electric motor is a device that converts electrical energy into mechanical energy.
Principle: It works on the principle that a current-carrying conductor placed in a magnetic field experiences
a force.
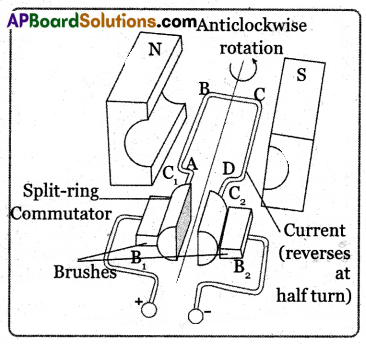
Working Procedure:
- It comprises an armature, a strong horseshoe-type magnet, split rings, and carbon brushes.
- Initially let the plane of the coil in a horizontal position.
- The split ring C touches brush B2 and split ring C2 touches brush B2 when the current flows in the direction ABCD as shown in the figure.
- According to the right-hand rule, no force acts on arm CB and DA because they are parallel to the magnetic field.
- The force acting on the arm AB pushes it downwards while the force acting on the arm CD pushes it upwards. So the armature rotates in the anti-clockwise direction.
- After half a rotation the split ring C1 comes in contact with brush B, and C2 in contact with brush B1. So the current in the coil is reversed and flows in the direction of DCBA.
- If the direction of the current in the coil is unchanged the coil gets to and fro motion.
- In an electric motor, the split rings act as a commutator which reverses the direction of the flow of current through a circuit.
- Now the coil rotates continuously in the anti-clockwise direction.
![]()
Question 16.
(A) Write how the N2 molecule forms. Explain.
(OR)
(B) For metals and ores write the examples for the following.
- Sulphate
- Carbonate
- Sulphide
- Halide
Answer:
(A) Formation of N2 Molecule:
- 7N has electronic configuration 1s2 2s2 2px1 2py1 2pz1.
- Suppose that the ‘px‘ orbital of one ‘N’ atom overlaps the ‘px‘ orbital of the other ‘N’ atom giving spx-px along the inter-nuclear axis.
- The py and pz orbitals of one ‘N’ atom overlap the py and pz orbital of the other ‘N’ atom laterally, respectively perpendicular to the inter-nuclear axis giving ppy-py and ppz-pz bonds.
- Therefore N2 molecule has a triple bond between two nitrogen atoms. (N≡N) with one sigma and two pi bonds.
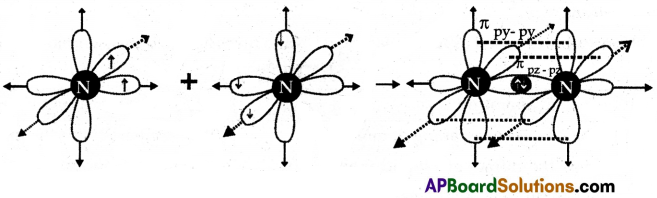
(B)
| Ores | Example | Formula | Metal |
| 1. Sulphate | Epsom Salt | MgSO4.7H2O | Mg |
| Gypsum | CaSO4.2H2O | Ca | |
| 2. Carbonate | Magnesite | MgCO3 | Mg |
| Limestone | CaCO3 | Ca | |
| 3. Sulphide | Zinc blende | ZnS | Zn |
| Cinnabar | HgS | Hg | |
| Galena | PbS | Pb | |
| 4. Halide | Horn Silver | AgCl | Ag |
| Rock Salt | NaCl | Na |
Question 17.
(A) Explain the procedure to prove Snell’s law.
(OR)
(B) Explain experimentally, how a metal reacts with an acid.
Answer:
(A) Aim: Obtaining a relation between the angle of incidence and the angle of refraction.
Materials Required: Pro circle, scale, small black painted plank, a semi-circular glass disc of thickness nearly 2 cm, pencil, and laser light.
Procedure:
(i) Take a wooden plank that is covered with the white chart.
(ii) Draw two perpendicular lines, passing through the middle of the paper as shown in the figure.
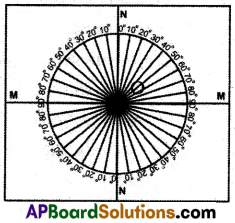
(iii) Let the point of intersection be O. Mark one line as NN which is normal to the other line marked as MM.
(iv) Here MM represents the line drawn along the interface of two media and NN represents the normal drawn to this line at ‘O’.
(v) Now place a semi-circular glass disc so that its diameter coincides with the interface line (MM) and its center coincides with point O.
(vi) Point a laser light along NN in such a way that the light propagates from air to glass through the interface at point O and observe the path of laser light coming from the other side of the disc.

(vii) Send laser light along a line that makes 15° (angle of incidence) with NN and see that it passes through point O. Measure its corresponding angle of refraction, by observing laser light coming from the other side (circular side) of the glass slab.
(viii) Note these values in the table. Do the same for the angles of incidence such as 20°, 30°, 40°, and 50° note the corresponding angles of refraction.

(ix) Find sin i, sin r for every ‘i’ and ‘r’ and evaluate sin i/sin r for every incident angle ‘i’.
(x) We get \(\frac{\sin i}{\sin r}\), a constant and this ratio is called refractive index.
(B) Aim: To know the reaction of acids with metals.
Materials Required: Test tube, delivery tube, glass trough, candle, soap water, dil. HCl and zinc granules.
Procedure:
(i) Take about 10 ml of dil. HCl acid in a test tube and add a few zinc granules to it.
(ii) We will observe the formation of gas bubbles on the surface of zinc granules.
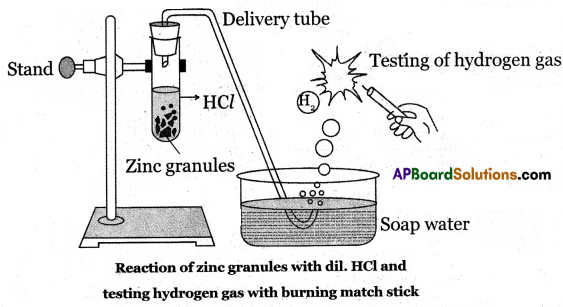
(iii) Pass the gas being formed through the soap solution taken in a trough using a delivery tube.
(iv) Gas-filled bubbles are formed in the soap solution which rises into the air
(v) Bring a burning candle near a gas-filled soap bubble. The gas present in soap bubbles bum with a pop sound and puts off the burning candle.
(vi) Only hydrogen gas bums make a pop sound.
(vii) From this activity we conclude that hydrogen gas evolved in the reaction of dil. HCl with Zn metal.
Metal + Acid → Salt + Hydrogen
Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)