Timed practice with TS 10th Class Physical Science Model Papers Set 9 is crucial for improving speed and efficiency during exams.
TS 10th Class Physical Science Model Paper Set 9 with Solutions
Time: 1 Hour 30 minutes
Maximum Marks: 40
General Instructions:
- Read the question paper and understand every question thoroughly and write answers in given 1.30 hrs. time.
- 3 very short answer questions are there in section – I. Each question carries 2 marks. Answer all the questions. Write answer to each question in 3 to 4 sentences.
- 3 short answer questions are there in section – II. Each question carries 3 marks. Answer all the questions. Write answer to each question in 5 to 6 sentences.
- 3 essay type answer questions are there in section – III. Each question carries 5 marks. Answer all the questions. Write answer to each question in 8 to 10 sentences. Internal choice is given in this section.
Part – A (30 Marks)
Section – I (3 × 2 = 6 Marks)
Instructions :
- 3 Very short answer questions are there in section – I.
- Answer ALL the questions. Each question carries 2 marks.
- Write answer to each question in 3 to 4 sentences.
Question 1.
Suggest a new use with a spherical mirror.
Answer:
- Concave mirrors are used in long focal length camera lenses.
- In ‘Super snooper’ to focus and magnify sound from a great distance onto a microphone pick up.
- At one end of some gas LASERS to focus the emerging beam of light.
- In the telescopes to focus the very weak radio waves onto the suspended antenna.
Question 2.
What happens, if the eye lens lose its ability for accommodation ?
Answer:
- If the eye lens lose its ability for accommodation, the vision becomes blurred.
- A person who lose thsi ability of eye lens may suffer from eye defects like myopia, hypermetropia and presbyopia.
![]()
Question 3.
Write about electrolysis of NaCl.
Answer:
- Fused NaCl is electrolysed with steel cathode and graphite anode.
- The metal sodium (Na) will be deposited at cathode and chlorine gas liberates at the anode.
At Cathode : 2 Na+ + 2e– → 2Na
At Anode : 2Cl– → Cl2 + 2e–
Section – II (3 × 3 = 9 Marks)
Instructions :
- 3 Short answer questions are there in section – II.
- Answer ALL the questions. Each question carries 3 marks.
- Write answer to each question in 5 to 6 sentences.
Question 4.
The atomic number of elements P, Q, R, S and T are given below :

From the above table, answer the following questions :
a) Which element among these is a non metal ?
b) Which element belongs to 3rd period of periodic table?
c) Which is an inert gas ?
d) Which two elements are chemically similar ?
Answer:
The electronic configuration of the elements are as follows :

a) Element P is a non-metal since it has five valence electrons.
b) Element R belongs to third period since it has three shells.
c) Element Q is an inert gas element since it has complete octet.
d) Elements R and S are chemically similar since they have same number of valence electrons.
Question 5.
Write about ‘Hydrogen bond’.
Answer:
- Hydrogen bond is formed between molecules in which hydrogen atom is attached to an atom
of an element with large electronegativity and very small size (F, O, N). Because of hydrogen bond the molecules associate themselves and hence possess higher B.P’s and M.P’s. - The hydrogen bond formed between two molecules is called inter-molecular hydrogen bond.
- The hydrogen bond formed between different groups of the same molecule is called intra-molecular hydrogen bond.
Question 6.
Write the molecular formula of the first four compounds of the homologous series of aldehydes.
Answer:
Homologous series of aldehydes are Formaldehyde, Acetaldehyde, Propionaldehyde and Butylaldehyde.
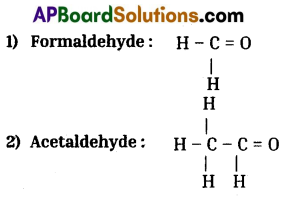

![]()
Section – III (3 × 5 = 15 Marks)
Instructions :
- 3 Essay answer questions are there in section.
- Answer ALL the questions. Each question carries 5 marks.
- Internal choice is given in this section.
- Write answer to each question in 8 to 10 sentences.
Question 7.
What are the materials required for the experiment to show the chemical decomposition of water ? Write the procedure of the experiment. Name the products which we get in this reaction.
(OR)
What is meant by “water of crystallization” of a substance ? Describe an activity to show the water of crystallization.
Answer:
Aim : To Show Chemical decomposition of water
Materials required for chemical decomposition of water :
A plastic mug, two carbon rods, two corks, two test tubes, connecting wires,
9 V battery, water and some drops of acid.
Procedure :
- Take a plastic mug, drill holes at the base.
- Fit two one holed rubber stoppers in these holes:
- Insert two carbon electrodes in these rubber stoppers.
- Connect the electrodes to 9 V battery.
- Fill the mug with water, so that the electrodes are immersed.
- Add few drops of any acid.
- Take two test tubes filled with water and insert them over the two carbon electrodes.
- Switch on the circuit and leave the apparatus undisturbed for some time.
- We notice the liberation of bubbles at both the electrodes. These bubbles displace the water in the test tubes.
- After the test tubes are filled with gas, take them out and test the gases with a burning matchstick.
The products which we get in this reaction: The gases are considered oxygen and hydrogen.
(OR)
Water of Crystallization : Water of crystallization is the fixed number of water molecules chemically attached to each formula unit of a salt in its crys¬talline form. The salts which contains water of crystallization are called hydrated salts.

Activity :
- Take a few crystals of copper sulphate in a dry test tube.
- Heat the crystals strongly by keeping the test tube over the flame of a burner for sometime.
- On heating, the blue coloured copper sulphate crystals turns white and a powdery substance is formed.
- We can also see tiny droplets of water in the test tube.
- Now cool the test tube and add 2 or 3 drops of water on the white copper sulphate powder formed above.
- The blue colour of copper sulphate crystals are reformed. They become blue again.
- From the above activity we conclude that some water molecules are fixed in the blue coloured copper sulfate crystals.
Question 8.
How will you calculate the focal length of a biconvex lens that is used to correct the defect of Hypermetropia ? Explain it mathematically.
(OR)
Collect the information about the scientists who developed the atomic theories.
Answer:
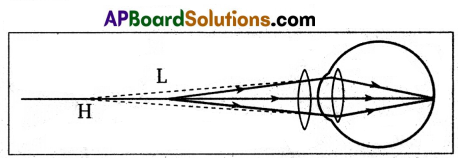
- Let us consider the object is at least distance of distinct vision (L).
- Hypermetropia is corrected when the image is formed at the near point H.
- This image acts like an object for the eye lens. Final image is formed at retina.
Here object distance (u) = -25cm (least distance of distinct vision)
image distance (v) = distance of near point (H) = -d
Let ‘f’ be the focal length of a bi-convex lens
Lens formula : \(\frac{1}{\mathrm{f}}=\frac{1}{\mathrm{v}}-\frac{1}{\mathrm{u}}\)
= \(\frac{1}{-d}-\frac{1}{-25}\)
= \(\frac{1}{-d}+\frac{1}{25}\)
⇒ \(\frac{1}{\mathrm{f}}=\frac{\mathrm{d}-25}{25 \mathrm{~d}}\) Hence f = \(\frac{25 d}{d-25}\) - From it if d > 25 cm, then f becomes +ve.
- We need to use biconvex lens to correct the defect of hypermetropia.
(OR)
- Atom is the smallest particle present in atom. Later it is proved proton (positive), electron (negative), neutron (neutral) are in atom.
- Thomson proposed plum pudding model and atom as a whole electrically neutral.
- Rutherford discovered nucleus and proposed that entire positive charge and mass of atom concentrate at nucleus and electrons are revolving around nucleus in different orbits.
- Bohr proposed that electrons revolving around nucleus in stationary orbits. As long as electron revolve in a particular stationary orbit its energy does not change.
- Quantum numbers finally explained the position of various sub-atomic particles that is electron, proton and neutron.
Question 9.
12 V battery is connected in a circuit and to this 4Ω, 12Ω resistors are cqnnected in parallel, 3Ω resistor is connected in series to this arrangement. Draw the electric circuit from this information and find the current in the circuit.
(OR)
A particle of charge 3.6 pC is projected into a uniform magnetic field of induction 5T with a speed of 2.5 × 105 m/s. Calculate the radius and time period of the particle taking its mass to be 1 gm.
Answer:
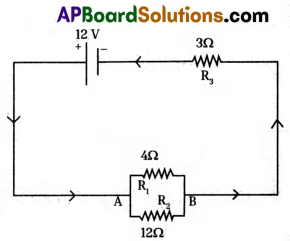
Potential difference (V) = 12 V
Equivalent resistance between AB is R then,
\(\frac{1}{\mathrm{R}}=\frac{1}{4}+\frac{1}{12}=\frac{3+1}{12}=\frac{4}{12}=\frac{1}{3}\)
R = 3 Ω
Equivalent resistance in the circuit
= R + 3 = 3 + 3 = 6Ω
Current in the circuit is I then I= \(\frac{V}{R}\)(∵ V = IR Ohm’s law)
= \(\frac{12}{6}\) = 2A.
∴ Current in the circuit (I) = 2A
(OR)
Mass of the particle : m = 1 gm = 10-3 kg
Charge on the particle : q = 3.6
μc = 3.6 × 10-6 C
Magnetic field induction : B = 5T
Speed of the particle : v = 2.5 × 105 m/s
Radius of the circular part : r = \(\frac{\mathrm{mv}}{\mathrm{bq}}=\frac{10^{-3} \times 2.5 \times 10^5}{5 \times 3.6 \times 10^{-6}}\)
r = \(\frac{10^8}{7.2}\) ⇒ r = 1.3889 × 107 ⇒ r = 1.4 × 107 m
Time period of rotation: T = 2π\(\frac{\mathrm{m}}{\mathrm{Bq}}\) = 2π\(\frac{10^{-3}}{5 \times 3.6 \times 10^{-6}}\)
T = \(\frac{\pi}{9}\) × 103 sec = 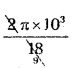 T = \(\frac{\pi}{9}\) × 103 sec
T = \(\frac{\pi}{9}\) × 103 sec
![]()
Part – B (10 × 1 = 10 Marks)
Instructions :
- Answer ALL the questions.
- Each question carries 1 mark.
- In this section there are 4 options (A / B / C / D) to each question. Choose the appropriate answer and write the answer in the brackets given against the question. Part – B must be attached to the answer booklet of Part – A.
Question 1.
In the case of …………….. mirror the magnification is always positive.
A) concave
B) plane
C) convex
D) all the above
Answer:
C) convex
Question 2.
A solution of potassium iodide reacts with lead nitrate to give ……………..
A) KNO3
B) PbI2
C) Both A and B
D) PbO
Answer:
C) Both A and B
Question 3.
Which of the following is produced by the action of chlorine on dry slaked lime?
A) Washing soda
B) Bleaching powder
C) Plaster of Paris
D) Baking soda
Answer:
B) Bleaching powder
Question 4.
Which of the following substances is used as an antichlor ?
A) CaOCl2
B) Na2S2O3
C) Na2SO4
D) CuSO4
Answer:
B) Na2S2O3
Question 5.
Which of the following precipitate is formed on mixing calcium hydroxide with carbon dioxide?
A) Red precipitate of calcium carbonate
B) Blue precipitate of calcium carbonate
C) White precipitate of calcium carbonate
D) Black precipitate of calcium carbonate
Answer:
C) White precipitate of calcium carbonate
Question 6.
The radius of curvature of a piano convex lens is doubled without changing the material of medium, it focal length will be
A) double
B) halved
C) remains same
D) insufficient data
Answer:
A) double
![]()
Question 7.
Find the refractive index of the glass which is a symmetrical convergent lens if its focal length is equal to the radius of curvature of its surface
A) 1
B) 2
C) 1.5
D) -1.5
Answer:
B) 2
Question 8.
The angle of vision for a healthy human being is about
A) 60°
B) 30°
C) 45°
D) 90°
Answer:
A) 60°
Question 9.
Which of the following elements is electro-negative?
A) Sodium
B) Oxygen
C) Magnesium
D) Calcium
Answer:
B) Oxygen
Question 10.
The process of obtaining the pure metal from the impure metal is called ……………. of the metal.
A) Liquation
B) Refining
C) Poling
D) Distillation
Answer:
B) Refining