Timed practice with TS 10th Class Physical Science Model Papers Set 7 is crucial for improving speed and efficiency during exams.
TS 10th Class Physical Science Model Paper Set 7 with Solutions
Time: 1 Hour 30 minutes
Maximum Marks: 40
General Instructions:
- Read the question paper and understand every question thoroughly and write answers in given 1.30 hrs. time.
- 3 very short answer questions are there in section – I. Each question carries 2 marks. Answer all the questions. Write answer to each question in 3 to 4 sentences.
- 3 short answer questions are there in section – II. Each question carries 3 marks. Answer all the questions. Write answer to each question in 5 to 6 sentences.
- 3 essay type answer questions are there in section – III. Each question carries 5 marks. Answer all the questions. Write answer to each question in 8 to 10 sentences. Internal choice is given in this section.
Part – A (30 Marks)
Section – I (3 × 2 = 6 Marks)
Instructions :
- 3 Very short answer questions are there in section – I.
- Answer ALL the questions. Each question carries 2 marks.
- Write answer to each question in 3 to 4 sentences.
Question 1.
Which objects at your home act as spherical mirrors ?
Answer:
- The outer surfaces of stainless steel utensils, spoons, plates etc., act as convex mirrors.
- The inner surfaces of the above said articles act as concave mirrors.
- Any bulged surface with high polish acts as a convex mirror.
Question 2.
Doctor advised to use 2D lens. What is its focal length ?
Answer:
Given that the power of lens P = 2D
We know that, P = \(\frac{100}{\mathrm{f}(\mathrm{cm})}\)
2 = \(\frac{100}{\mathrm{f}}\); 2f = 100
f = \(\frac{100}{2}\) = 50 cm
∴ The focal length of the lens = 50 cm
![]()
Question 3.
What is 22 carat gold ? Why it is preferred for making jewellery ?
Answer:
- Pure gold, known as 24 carat gold is very soft.
- So it is not. suitable for making jewellery.
- So they use 22 carat gold in which pure gold is alloyed with 2 parts of either silver or copper for making gold jewellery.
Section – II (3 × 3 = 9 Marks)
Instructions :
- 3 Short answer questions are there in section – II.
- Answer ALL the questions. Each question carries 3 marks.
- Write answer to each question in 5 to 6 sentences.
Question 4.
How many elements are there in 1st period ? Why ?
Answer:
- The first period contains only two elements.
- The first period starts with k-shell, which is the first main shell.
- It contains only one sub-shell that contains two types of electronic configurations.
- So there are only two elements that are having electronic configuration as 1s1 and 1s2.
Question 5.
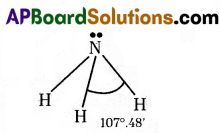
“Bond angle of ammonia reduced to 107°48’ from 109°28′” is said Ramya. Is she correct ? Justify your answer.
Answer:
Yes. She is correct.
Justification :
- In NH3 there are three bond pairs (N – H) and one lone pair of electrons (N) is present around the central atom of nitrogen.
- So, the lone pair electron shows repulsion on bond pairs.
- Hence, to minimize the repulsion force bond angle changes from 109°28’ to 107°48’.
- At the same time the shape also changes from tetrahedral to pyramidal.
Question 6.
What is 22 carat gold ? Why it is preferred for making jewellery ?
Answer:
- Pure gold, known as 24 carat gold is very soft.
- So it is not suitable for making jewellery.
- So they use 22 carat gold in which pure gold is alloyed with 2 parts of either silver or copper for making gold jewellery.
Section – III (3 × 5 = 15 Marks)
Instructions :
- 3 Essay answer questions are there in section.
- Answer ALL the questions. Each question carries 5 marks.
- Internal choice is given in this section.
- Write answer to each question in 8 to 10 sentences.
Question 7.
Balance the following chemical equations.
i) Zn(s) + AgNO3(aq) → Zn (NO3)2(aq) + Ag(s)
ii) Fe2O3(s) + C(s) → Fe(s) + CO2(g)
iii) Ag + H2S + O2 → Ag2S + H2O
iv) Cu(s) + O2(g) → CUO(s)
(OR)
List out the materials for the experiment “when” Hydrochloric acid reacts with NaHCO3 and evolves CO2”. Write the experiment procedure.
Answer:
i) Zn(s) + AgNO3(aq) → Zn(NO3)2(aq) + Ag(s)
Step – I: Zn + 2 AgNO3 → Zn (NO3)2 + Ag
Step – II: Zn(s) + 2 AgNO3(aq) → Zn (NO3)2(aq) + 2Ag(s)
ii) Fe2O3(s) + C(s) → Fe(s) + CO2(g)
Step – I: Fe2O3 + C → 2 Fe + CO2
Step – II: 2Fe2O3 + C → 2 Fe + 3 CO2
Step – III: 2Fe2O3(s) + 3C(s) → 4 Fe(s) + 3 CO2(g)
iii) Ag(s) + H2S(s) + O2(g) → Ag2S(s) + H2O(l)
Step – I: 2 Ag+ H2S + O2 → Ag2S + 2 H2O
Step – II: 2 Ag + 2H2S + O2 → Ag2S + 2 H2O
Step – III: 2 Ag + 2H2S + O2 → 2Ag2S + 2 H2O
Step – IV: 4 Ag(s) + 2H2S(g) + O2(g) → 2Ag2S(s) + 2 H2O(l)
iv) CU(s) + O2(g) → CUO(s)
Step – I: Cu + O2 → 2CuO
Step – II: 2Cu(s) + O22(g) → 2CuO(s)
(OR)
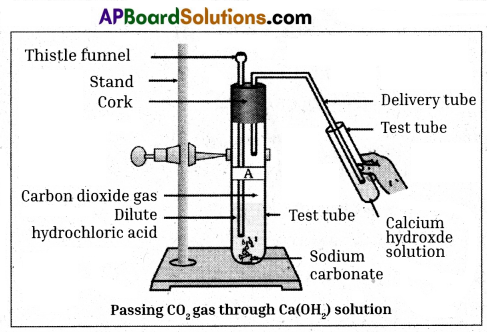
Material required : Stand; two hold rubber cork, two test tubes, thistle funnel, delivery tube, Ca(OH)2, Na2CO3, HCl.
Procedure :
- Take two test tubes, label them as A and B.
- Take about 0.5 gm of sodium carbonate – (Na2CO3) in test tube A and about 0.5 gm of sodium hydrogen carbonate (NaHCO3) in test tube B.
- Add about 2ml of dilute HCl to both the test tubes.
- Pass the gas produced in each case through lime water and record your observations.
- We observe that the lime water turns to milky white.
- The reactions are
Na2CO3(s) + 2HCl(aq) → 2NaCl(aq) + H2O(l) + CO2(g)
NaHCO3(s) + HCl(aq) → NaCl(aq) + H2O(l) + CO2(g)
Reactions when the gas is passed through lime water.
Ca(OH)2(aq) + CO2(s) → CaCO3 ↓+ H2O(l)
(white precipitate)
On passing excess carbon dioxide, the following reaction takes place.
CaCO3(s) + H2O(l) + CO2(s) → Ca(HCO3)2(aq) (Soluble in water)
Thus from the above activity we can conclude that the reaction of metal carbonates and hydrogen carbonates with acids give a corresponding salt, carbon dioxide and water.
![]()
Question 8.
Refractive index of a lens is 1.5. When an object is placed at 30 cm, image is formed at 20 cm. Find its focal length. Which lens is it ? If the radii of curvature are equal then what is its value ?
(OR)
1s2 2s2 2p3 3s2 3p6 3d10 4s1 is the electronic configuration of Cu (Z = 29) which rule is violated while writing this configuration ? What might be the reason for writing this configuration ?
Answer:
Object distance u = – 30 cm (Infront of the lens)
Image distance v = 20 cm
From len’s formula = \(\frac{1}{\mathrm{f}}\) = \(\frac{1}{\mathrm{v}}\) – \(\frac{1}{\mathrm{u}}\) = \(\frac{1}{20}\) + \(\frac{1}{30}\)
⇒ f = 12 cm
The value of the focal length of the lens we get is positive so the lens is convex type.
For convex lens, Len’s maker’s formula is
\(\frac{1}{\mathrm{f}}\) = (1 – n) (\(\frac{1}{\mathrm{R}_1}+\frac{1}{\mathrm{R}_2}\))
R1 = R2 = R
\(\frac{1}{12}\) = (1.5 – 1)(\(\frac{2}{R}\))
\(\frac{1}{12}\) = 0.5 × \(\frac{2}{R}\) ⇒ R = 12 cm
∴ Radius of curvature is 12 cm.
(OR)
- The predicted electronic configuration of Cu (Z = 29) is 1s2 2s2 2p6 3s2 2p6 3s2 3p6 4s2 3d9.
- But experimentally it has been showed that the actual configuration is 1s2 2s2 2p6 3s2 3p6 4s1 3d10.
- Here Aufbau principle is violated. According to Aufbau principle, the electron occupies the orbital having least energy first.
- That means in the ground state the electronic configuration can be built up by placing electrons in the lowest available orbitals until the total number of electrons added is equal to the atomic number.
- But the atoms with half filled or fully filled orbitals in their outermost orbit are more stable
when compared with their neighbouring elements. - In order to get fully filled orbitals in the outermost orbit in copper the electrons in 4s and 3d re-distribute their energies to attain stability.
- Hence the actual electronic configuration of copper is Cu (Z = 29) [Ar] 4s1 3d10
Question 9.
What are the factors affecting the resistance of an electric conductor ? Explain any two factors.
(OR)
Collect information about material required and procedure of making a simple electric motor from internet and make a simple motor on your own. (Or) Make a simple electric motor on your own with the help of internet information about simple electric motor for material; procedure.
Answer:
The factors affecting the resistance of an electrical conductor are
- Nature of material
- Temperature
- Length of the conductor
- Area of cross section of conductor
Explanation:
- As the temperature increases the resistance increases and vice versa.
- As the material changes resistance changes.
- Resistance(R) is directly proportional to length(l) of the conductor (if T and A are kept constant) R ∝ l
- Resistance(R) is inversely proportional to area of cross section (A) (if l and T are kept constant)
R ∝ \(\frac{l}{\mathrm{~A}}\)
(OR)
Aim : Preparation of a simple electric motor.
Materials required : A wire of nearly 15 cm, 1.5V battery, iron nail, strong magnet paper clip.
Procedure :
- Attach the magnet to the head of the iron nail.
- Attach a paper clip to the open end of the magnet.
- Now attach the other end of the nail (Free end) to the cap (positive terminal) of the battery.
- Now connect the negative terminal of the battery and the head of the iron nail through a wire.
- We observe that the paper clip rotates.
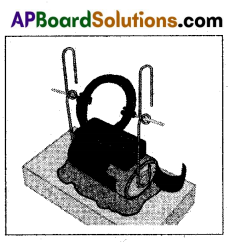
Another model :
Materials required : 1.5 m enamelled copper wire (about 25 gauge), 2 safety pins, 1.5V battery, magnets, rubber bands or bands cut from cycle tube.
Procedure:
- Wind copper wire on the battery nearly 10 15 turns to make a coil.
- Remove the coil and fix the ends as shown in the figure.
- Scape the insulation completely on one end of the coil.
- Scape the insulation on top, left and right of the other end. The bottom should be insulated.
- Now complete the electric motor as shown in the figure.
- We observe the rotation of coil.
Part – B (10 × 1 = 10 Marks)
Instructions :
- Answer ALL the questions.
- Each question carries 1 mark.
- In this section there are 4 options (A / B / C / D) to each question. Choose the appropriate answer and write the answer in the brackets given against the question. Part – B must be attached to the answer booklet of Part – A.
Question 1.
Which of the following image cannot be caught on the screen ?
A) real
B) lateral inversion
C) virtual
D) none of these
Answer:
C) virtual
Question 2.
The metal formed when zinc reacts with AgNO3 is
A) Au
B) Ag
C) Al
D) Cu
Answer:
B) Ag
Question 3.
Which of the following is slightly soluble in water ?
A) Ca(OH)2
B) KOH
C) Mg(OH)2
D) Be(OH)2
Answer:
D) Be(OH)2
Question 4.
Which of the following acid is injected by stinging hair of leaves of nettle plant that causing burning pain?
A) Methanoic acid
B) Hydrochloric acid
C) Nitric acid
D) Sodium thiosulphate
Answer:
A) Methanoic acid
![]()
Question 5.
Which of the following acid is produced by human’s stomach ?
A) CH3COOH
B) HCl
C) H2SO4
D) HNO3
Answer:
B) HCl
Question 6.
Name of the following lens.

A) Concave lens
B) Convex lens
C) Plano concave
D) Plano convex
Answer:
D) Plano convex
Question 7.
![]() denotes which lens ?
denotes which lens ?
A) Convex
B) Concave
C) Plano convex
D) Plano concave
Answer:
B) Concave
Question 8.
The human eye can focus objects at different distances by adjusting the focal length of the eye lens. This is due to
A) Presbyopia
B) Accommodation
C) Near sightedness
D) Far-sightedness
Answer:
B) Accommodation
Question 9.
What type of oxide would Ekaaluminium form ?
A) E3O2
B) E2O3
C) EO3
D) EO
Answer:
B) E2O3
Question 10.
Steel is an alloy of …………..
A) Fe and Zn
B) Fe and C
C) Cr and Fe
D) Zn and Cr
Answer:
B) Fe and C