Timed practice with TS 10th Class Physical Science Model Papers Set 5 is crucial for improving speed and efficiency during exams.
TS 10th Class Physical Science Model Paper Set 5 with Solutions
Time: 1 Hour 30 minutes
Maximum Marks: 40
General Instructions:
- Read the question paper and understand every question thoroughly and write answers in given 1.30 hrs. time.
- 3 very short answer questions are there in section – I. Each question carries 2 marks. Answer all the questions. Write answer to each question in 3 to 4 sentences.
- 3 short answer questions are there in section – II. Each question carries 3 marks. Answer all the questions. Write answer to each question in 5 to 6 sentences.
- 3 essay type answer questions are there in section – III. Each question carries 5 marks. Answer all the questions. Write answer to each question in 8 to 10 sentences. Internal choice is given in this section.
Part – A (30 Marks)
Section – I (3 × 2 = 6 Marks)
Instructions :
- 3 Very short answer questions are there in section – I.
- Answer ALL the questions. Each question carries 2 marks.
- Write answer to each question in 3 to 4 sentences.
Question 1.
Write about various distances related to mirrors.
Answer:
The various distances related to mirrors are :
- Focal Length (f) : The distance between vertex and focus is called focal length.
- Radius of curvature (R) : The distance between vertex and centre of curvature is called radius of curvature.
- Object distance : It is the distance between the object and pole of mirror and is denoted by ‘u’.
- Image distance : It is the distance between the image and pole of the mirror and is denoted by ‘v’.
Question 2.
Why do we use lenses in spectacles to correct defects of vision?
Answer:
- The process of adjusting focal length is called “accommodation”.
- This process has to be done by eye itself.
- Sometimes the eye may gradually lose its power of accommodation. In such condition the person cannot be able to see the object clearly and comfortably.
- In this situation we have to use lenses in spectacles to correct defects of vision.
![]()
Question 3.
How do various metals in activity series react with dilute strong acids ?
Answer:
- The metals from potassium to lead displace hydrogen from dilute strong acids with decreasing reactivity.
a) The reaction of potassium is explosive.
b) The reaction ofmagnesium is vigorous.
c) The reaction of iron is steady.
d) The reaction of lead is slow. - The metals from copper to gold do not displace H2 from strong dilute acids.
Section – II (3 × 3 = 9 Marks)
Instructions :
- 3 Short answer questions are there in section – II.
- Answer ALL the questions. Each question carries 3 marks.
- Write answer to each question in 5 to 6 sentences.
Question 4.
The electronic configuration of atom A is 2, 8, 6
a) What is the atomic number of element A ?
b) State whether the atomic size of element A is bigger or smaller than the atom having atomic number 14. Why ?
c) Which of the elements exhibit similarity in chemical properties as element A O(8), C(6), N(7), AV(18). Why ?
d) How the element is formed inert gas configuration ?
Answer:
The electronic configuration of atom – A is 2, 8, 6.
a) Atomic number of element‘A is 16. i.e,, Sulphur.
b) The atom which having atomic number -14 is Silicon (Si).
Atomic size of element decreases across period from left to right. So. the atomic size of element ‘A’ is smaller than the atom having atomic number 14.
c) Element oxygen O8 – exhibit similarity in chemical properties as element A, because they belong to the same group.
d) Given element – A becomes inert gas i.e., Argon configuration by gaining ‘2’ electrons.
Question 5.
What is Hybridisation ? Explain the formation of BeF2 molecule using hybridisation.
Answer:
Hybridisation: It is a phenomenon of inter mixing of atomic orbitals of almost equal energy which are present in the outer shells of the atom and their reshuffling or redistribution into the same number of orbitals but with equal properties like energy and shape.
This process is called hybridisation.
BeF2 does not exist. Because valency of Be is 2 and the valency of F is 3. It means there are no equal energy orbitals in outer shells of Be and F.
Question 6.
How many isomers can be drawn for pentane with molecular formula C5H12 ? What are they ? Draw their structures and mention their common names.
Answer:
Isomers of pentane are three.
There are :
- Pentane
- Isopentane
- Neo pentane
Structures :
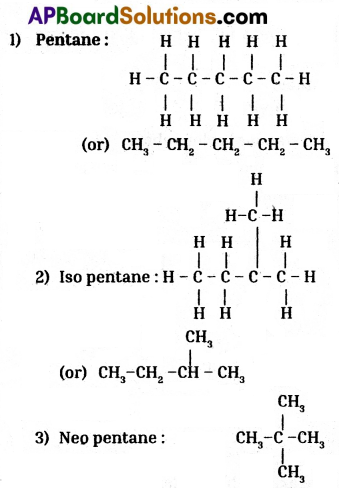
![]()
Section – III (3 × 5 = 15 Marks)
Instructions :
- 3 Essay answer questions are there in section.
- Answer ALL the questions. Each question carries 5 marks.
- Internal choice is given in this section.
- Write answer to each question in 8 to 10 sentences.
Question 7.
Balance the following chemical equation after writing the symbolic representation.
a) Magnesium(s) + Hydrochloric acid(aq) → Magnesium chloride(aq) + Hydrogen(g)
b) Zinc(s) + Calcium chloride(aq) → Zinc Chloride(aq) + Calcium(s)
(OR)
Raghu wants to test the pH of given samples by using universal indicator. What is the procedure that he follows?
Answer:
A) a) Magnesium(s) + Hydrochloric acid(aq) → Magnesium
1) Write the unbalanced equation using correct chemical formula for all sub-stances.
Mg(s) + HCl(aq) → MgCl2(aq) + H2(g)
2) Balancing the atoms of each element on both sides.
1 Mg(s) + 2 HCl(aq) → 1 MgCl2(aq) + 1 H2(g)
3) Write the co-efficient of smallest ratio.
1 Mg(s) + 2 HCl(aq) → 1 MgCl2(aq) + 1 H2(g)
4) The above equation is balanced.
Mg(s) + HCl(aq) → MgCl2(aq) + H2(g)
b) Zinc(s) + Calcium chloride(aq) → Zinc Chloride(aq) + Calcium(s)
1) Write the unbalanced equation using correct chemical formula for all substances.
Zn(s) + CaCl2(aq) → ZnCl2(aq) + Ca(s)
2) Balancing the atoms of each element on both sides.
1 Zn(s) + 1 CaCl2(aq) → 1 ZnCl2(aq) + 1 Ca(s)
3) Write the co-efficient of smallest ratio.
1 Zn(s) + 1 CaCl2(aq) → 1 ZnCl2(aq) + 1 Ca(s)
4) The above equation is balanced.
Zn(s) + CaCl2(aq) → ZnCl2(aq) + Ca(s)
(OR)
B) To test the pH of given samples, Raghu has to follow the below procedure.
- He has to take five clean test tubes in a test tube stand and label A, B, C, D and E to these tubes.
- He has to pour 5 ml of given samples in each test tube.
- He has to add two drops of universal indicator to each test tube.
- After changing the colour he has to compare with standard colour chart.
- The chart gives approximate value of pH of the concerned sample.
- The observations to be recorded and finally he may confirm the nature of the given samples.
Question 8.
Derive expression for lens maker’s formula. (Or) Prove \(\frac{1}{\mathrm{f}}\) = (n – 1) )(\(\frac{1}{R_1}-\frac{1}{R_2}\))
(OR)
1s2 2s2 2p6 3s2 3p6 3d10 4s1 is the electronic configuration of Cu (Z = 29) which rule is violated while writing this configuration ? What might be the reason for writing this configuration ?
Answer:
A) Procedure:
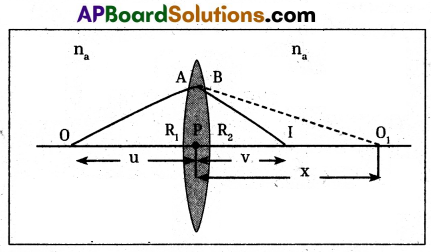
- Imagine a point object ‘O’ placed on the principal axis of the thin lens.
- Let this lens be placed in a medium of refractive index na and let refractive index of lens be nb.
- Consider a ray, from ‘O’ which is incident on the convex surface of the lens with radius of curvature R1 at A.
- The incident ray refracts at A.
- It forms image at Q, if there were no concave surface.
- From figure Object distance PO = – u;
Image distance PQ = v = x
Radius of curvature R = R1
n1 = na and n2, = nb.
Substituting the values in
We have \(\frac{n_2}{v}-\frac{n_1}{u}=\frac{\left(n_2-n_1\right)}{R}\) ……… (1) - But the ray suffers another refraction at B on the concave surface with radius of curvature
(R2) - At B the ray is refracted and reaches I.
- The image Q of the object due to the convex surface. So I is the image of Q for concave surface.
- Object distance u = PQ = +x
Image distance PI = v
Radius of curvature R = – R2 - The refraction the concave surface of lens is medium -1 and surrounding is medium -2.
∴ n1 = nb and n2 = na
Substituting values in
\(\frac{\mathrm{n}_2}{\mathrm{v}}-\frac{\mathrm{n}_1}{\mathrm{u}}=\frac{\left(\mathrm{n}_2-\mathrm{n}_1\right)}{\mathrm{R}} \frac{\mathrm{n}_{\mathrm{a}}}{\mathrm{v}}-\frac{\mathrm{n}_{\mathrm{b}}}{\mathrm{x}}=\frac{\left(\mathrm{n}_{\mathrm{b}}-\mathrm{n}_{\mathrm{a}}\right)}{\mathrm{R}_2}\) …………. (2)
Adding (1) and (2) and dividing both sides by na we have
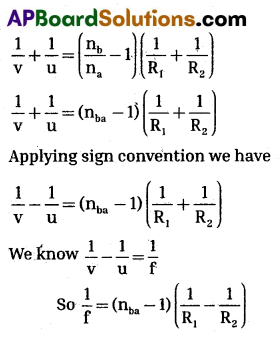
If the surrounding medium is air we have ba = n (absolute refractive index)
∴ \(\frac{1}{\mathrm{f}}\) = (n – 1) )(\(\frac{1}{R_1}-\frac{1}{R_2}\))
This is called lens maker’s formula.
(OR)
B)
- The predicted electronic configuration of Cu (Z = 29) is 1s2 2s2 2p6 3s2 2p6 3s2 3p6 4s2 3d9.
- But experimentally it has been showed that the actual configuration is 1s2 2s2 2p6 3s2 3p6 4s1 3d10.
- Here Aufbau principle is violated. According to Aufbau principle, the electron occupies the orbital having least energy first.
- That means in the ground state the electronic configuration can be built up by placing electrons in the lowest available orbitals until the total number of electrons added is equal to the atomic number.
- But the atoms with half filled or fully filled orbitals in their outermost orbit are more stable when compared with their neighbouring elements.
- In order to get fully filled orbitals in the outermost orbit in copper the electrons in 4s and 3d re-distribute their energies to attain stability.
- Hence the actual electronic configuration of copper is Cu (Z = 29) [Ar] 4s13d10
![]()
Question 9.
A circuit is made with a copper wire as shown in the diagram. We know that conductor’s resis¬tance is directly proportional to its length. Calculate the equivalent resistance between points 1 and 2.
(OR)
Explain the working process of induction stove.
Answer:
A) Let the resistance of the wire be ‘R’ and length of the wire be ‘l’.
The shape of the circuit be square length of the side (l) = R
In a square diagonal is √2 times its length = √2l
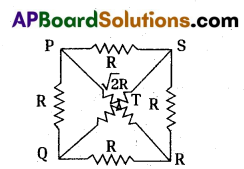
Resistance towards diagonal is √2R
The circuit diagrams for the given arrangement is along PTR and QTS is in effective as no current flows through it.

PQ and PS are in series so, effective resistance are R1 + R2 = R + R = 2R.
QR and SR are in series so, effective resistance are R1 + R2 = R + R = 2R
Redrawn of the circuit again as resultant resistance between the points 1 and 2 is,
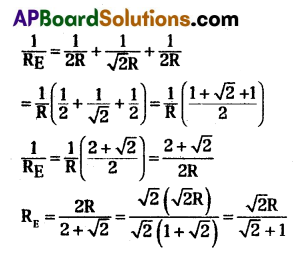
(OR)
B)
- An induction stove works on the principle of electromagnetic induction.
- A metal coil is kept just beneath the cooling surface. It carries alternating current (AC). So that AC produces an alternating magnetic field.
- When you keep a metal pan with water on it, the varying magnetic field beneath it crosses the bottom surface of the pan and EMF is induced in it.
- Since pan has a finite resistance, the flow of induced current in it produces heat and this heat is conducted to the water.
- That’s why we call this stove as induction stove.
Part – B (10 × 1 = 10 Marks)
Instructions :
- Answer ALL the questions.
- Each question carries 1 mark.
- In this section there are 4 options (A / B / C / D) to each question. Choose the appropriate answer and write the answer in the brackets given against the question. Part – B must be attached to the answer booklet of Part – A.
Question 1.
Which mirrors are used in many vehicles as rearview mirror and window glasses of a car?
A) convex
B) bi convex
C) bi-concave
D) concave
Answer:
A) convex
Question 2.
The formula of hypochlorous acid is
A) HCl
B) H2SO4
C) HOCl
D) H2Cl3
Answer:
C) HOCl
Question 3.
The reaction of an acid with a base to give a salt and water is known as
A) Neutralization
B) Reduction
C) Oxidation
D) Crystallisation
Answer:
A) Neutralization
Question 4.
Which one of the following is weak acid ?
A) HNO3
B) CH3COOH
C) HCl
D) H2SO4
Answer:
B) CH3COOH
Question 5.
If the pH value of a solution is 12, then the character of solution of the solution is ( )
A) Base
B) Strong acid
C) Acid
D) Strong base
Answer:
D) Strong base
Question 6.
The focal length of the piano convex lens is 2R where R is the radius of curvature of the surface. Then the refractive index of the material of the lens is
A) \(\frac{3}{2}\)
B) \(\frac{5}{2}\)
C) \(\frac{2}{5}\)
D) \(\frac{2}{5}\)
Answer:
C) \(\frac{2}{5}\)
![]()
Question 7.
If the object is placed beyond the centre of curvature (C2) on the principal axis, then the image is formed ……………
A) between F1 and C1
B) beyond C1
C) ‘C’
D) at infinity
Answer:
B) beyond C1
Question 8.
Rainbow is due to
i) scattering of light
ii) refraction of light
iii) dispersion
iv) total internal reflection of light
A) i and iii
B) i and ii
C) iii and iv
D) i, ii and iv
Answer:
C) iii and iv
Question 9.
Elements belonging to the same period have
A) Same valency electrons
B) Same number of shells
C) Same atomic size
D) Same electron affinity
Answer:
B) Same number of shells
Question 10.
Magnetite is an ore of
A) Hg
B) Ag
C) Mg
D) Fe
Answer:
D) Fe