Timed practice with TS 10th Class Physical Science Model Papers Set 3 is crucial for improving speed and efficiency during exams.
TS 10th Class Physical Science Model Paper Set 3 with Solutions
Time: 1 Hour 30 minutes
Maximum Marks: 40
General Instructions:
- Read the question paper and understand every question thoroughly and write answers in given 1.30 hrs. time.
- 3 very short answer questions are there in section – I. Each question carries 2 marks. Answer all the questions. Write answer to each question in 3 to 4 sentences.
- 3 short answer questions are there in section – II. Each question carries 3 marks. Answer all the questions. Write answer to each question in 5 to 6 sentences.
- 3 essay type answer questions are there in section – III. Each question carries 5 marks. Answer all the questions. Write answer to each question in 8 to 10 sentences. Internal choice is given in this section.
Part – A (30 Marks)
Section – I (3 × 2 = 6 Marks)
Instructions :
- 3 Very short answer questions are there in section -1.
- Answer ALL the questions. Each question carries 2 marks.
- Write answer to each question in 3 to 4 sentences.
Question 1.
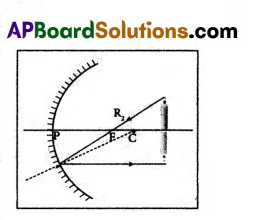
Why concave and convex mirrors are called spherical mirrors ?
Answer:
Concave and convex mirrors are called spherical mirrors because :
- The reflecting surfaces of concave and convex mirrors are considered to form a part of a hollow sphere.
- Their reflecting surface is not a plane surface.
Question 2.
How do you appreciate the working of “Retina”?
Answer:
- It is the innermost delicate membrane having a large number of receptors called ‘rods’ and ‘cones’.
- The rods identify the colour and the cones identify the intensity of light.
- The retina is a part on which the image of an object is formed
Question 3.
Give an example of auto reduction of sulphide ores.
Answer:
In the extraction of Cu, from its sulphide ore, the ore is subjected to partial roasting in air to give its oxide.
2Cu2S (S) + 3O2 (g) → 2Cu2O (S) + 2SO2 (g)
When the supply of air is stopped and the temperature is raised, the rest of the sulphide reacts with oxide and forms the metal and SO2.
2Cu2O (S) + Cu2S (S) ![]() 6Cu (S) + SO2(g)
6Cu (S) + SO2(g)
Section – II (3 × 3 = 9 Marks)
Instructions :
- 3 Short answer questions are there in section – II.
- Answer ALL the questions. Each question carries 3 marks.
- Write answer to each question in 5 to 6 sentences.
![]()
Question 4.
Negative ion of an element has bigger size than its neutral atom. Explain with an example.
Answer:
- When we take chlorine (Cl) atom, its electronic configuration is 1s2 2s2 2p6 3s2 3p5, which has 17 electrons and 17 protons.
- The electronic configuration of chloride ion is 1s2 2s2 2p6 3s2 3p6. Which has 18 electrons and 17 protons.
- Therefore, the nuclear attraction is less in Cl– ion when compared with chlorine atom.
- So, the size of chlorine atom is less than Cl– ion.
Question 5.
If the electronic configurations of atoms A and B are 1s2, 2s2, 2p6, 3s2, 3p1 and 1s2, 2s2, 2p4 respectively, then
a) Which atom forms negative ion ?
b) Which atom forms positive ion ?
c) What is the valency of atom A ?
d) What is the molecular formula of the compound formed by atoms A and B ?
Answer:
Given electronic configuration of atom A is 1s2 2s2 2p6 3s2 3p1 i.e., Alu-minium and B is 1s2 2s2 2p4i.e., Carbon.
a) The atom ‘B’ tends to form negative ion by gaining two electrons order to get nearest inert gas neon configuration is 1s2 2s2 2p6.
b) The atom ‘A’ tends to form positive ion by losing three electrons in order to get nearest inert gas. Neon configuration is 1s2 2s2 2p6.
c) Valency of atom‘A’ is ‘3’.
d) According to Criss-Cross method, the molecular formula of the corn-pound formed by atoms both A and B is A2B3 i.e., Al2O3.
Question 6.
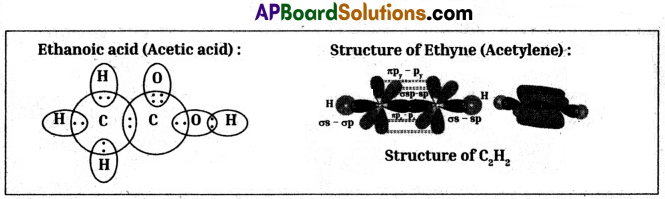
Draw the electron dot structures of Ethanoic acid and Ethyne (Acetylene).
Answer:
Section – III (3 × 5 = 15 Marks)
Instructions :
- 3 Essay answer questions are there in section.
- Answer ALL the questions. Each question carries 5 marks.
- Internal choice is given in this section.
- Write answer to each question in 8 to 10 sentences.
Question 7.
Explain the steps involved in balancing a chemical equation with an example.
(OR)
Write the chemical equation of preparation of baking soda. What are the uses of baking soda ?
Answer:
A chemical equation in which the number of atoms of different elements on the reactants side are same as those on product side is called a balanced equation.
Steps involved in balancing a chemical reaction: Let us consider the combustion reaction of Propane.
Step 1 : Write the unbalanced equation using correct chemical formula for all substances.
C3H8 + O2 → CO2 + H2O (Skeleton equation) .
Step 2 : Compare number of atoms of each element on both sides.
| Atom | No. of atoms in LH.S. | No. of atoms in RH.S. |
| C | 3((in C3H3) | 1 (in CO2) |
| H | 8 (in C3H3) | 2 (in H2O) |
| O | 2 (in O2) | 3 (in CO2, H2O) |
Find the co-efficients to balance the equation. In this case, there are 3 carbon atoms on the left side of the equation but only one on the right side. If we add a co-efficient of 3 to CO2 on the right side the carbon atoms balance.
C3H8 + O2 → 3 CO2 + H2O
Now, look at the number of hydrogen atoms. There are 8 hydrogen atoms On the left but only 2 on the right side. By adding a co-efficient of 4 to the H2O on the right side, the hydrogen atoms get balanced.
C3H8 + O2 → 3 CO2 + 4 H2O
Finally, look at the number of oxygen atoms. There are 2 on the left side but 10 on the right side. By adding a co-efficient of 5 to the 02 on the left side, the oxygen atoms get balanced.
C3H8 + 5 O2 → 3 CO2 + 4 H2O
Step 3 : Make sure the co-efficients are reduced to their smallest whole number values. The above equation is already with the co-efficients in smallest whole numbers. There is no need to reduce its co-efficients. Hence the final equation is
C3H8 + 5 O2 → 3 CO2 + 4 H2O
Step 4 : Check the answer. Count the numbers and kinds of atoms on both sides of the equation. to make sure they are the same.
(OR)
Preparation of baking soda:
- The chemical name of baking soda is sodium hydrogen carbonate and the formula is NaHCO,
- It is prepared as follows.
NaCl + H2O + CO2 + NH3 → NH4Cl + NaHCO3
Uses of baking soda:
- Baking soda is added for faster cooking.
- It is used as an ingredient in antacids.
- It is also, used in soda – acid fire extinguishers.
- It acts as a mild antiseptic.
- Baking powder can be prepared as a mixture of baking soda and a mild edible acid such as tartaric acid.
- Baking powder is used in preparation of bread or cake to make them soft and spongy.
![]()
Question 8.
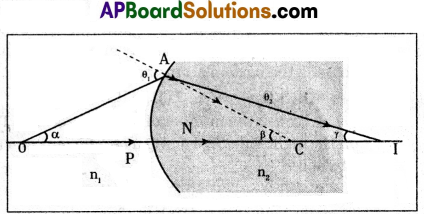
Find the relation among refractive indices of media, object distance, imagfe distance and radius of curvature.
(OR)
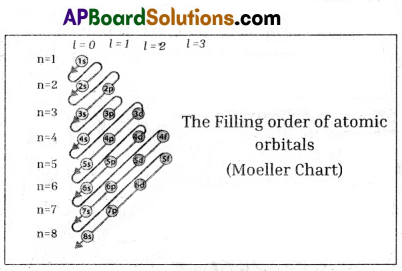
Draw Moeller chart of filling order of atomic orbitals. (Or) Draw a diagram showing the increasing value of (n + 1) of orbitals.
Answer:
1) Consider a curved surface separating two media of refractive indices n1 and n2 respectively.
2) O = Point object on the principal axis.
3) The ray, which forms an angle a with principal axis, meets the curved surface at A.
θ1 = The angle of incidence.
4) The ray bends and passes through the second medium along the line
AI = Refraction ray in 2nd medium.
5) θ2 = The angle of refraction.
6) I = The image is formed there.
7) γ = The angle made by the refracted ray AI with principal axis,
b = The angle between the normal and principal axis.
8) In the above figure
PO = the object distance = ‘u’
PI = image distance = ‘v’
PC = radius of curvature = ‘R’
n1, n2 are refractive indices of two media.
In a triangle ACO, θ1 = α + β
And in a triangle ACI, β = θ2 + γ
……. β – γ = θ2
According to Snell’s law,
we know n1 sin θ1 = n1 sin θ2
Substituting the values of θ1 and θ2,
we get, n1 sin (a + b) = n2 sin (β – γ ) ………… (1)
If the angles α, β and γ very small,
sin (α + β) = α + β and sin (β – γ) = β – γ
… n1(α + β) = n2(β – γ)
… n1α + n1β = n2β – n2γ ………….. (2)
Since all angles are small,
tan α = α = \(\frac{\mathrm{AN}}{\mathrm{NO}}\); tan β = β = \(\frac{\mathrm{AN}}{\mathrm{NC}}\) ; tan γ = γ = \(\frac{\mathrm{AN}}{\mathrm{NI}}\)
Substitute these values in equation (2),
⇒ n1\(\frac{\mathrm{AN}}{\mathrm{NO}}\) + n1\(\frac{\mathrm{AN}}{\mathrm{NC}}\) = n2\(\frac{\mathrm{AN}}{\mathrm{NC}}\) – n1\(\frac{\mathrm{AN}}{\mathrm{NI}}\) ……….. (3)
As the rays move very close to principal axis, the point N coincides with pole of the interface (P).
∴ NI = PI, NO = PO, NC = PC
Substituting those values in eqation (3),
⇒ \(\frac{\mathrm{n}_1}{\mathrm{PO}}+\frac{\mathrm{n}_1}{\mathrm{PC}}=\frac{\mathrm{n}_2}{\mathrm{PC}}-\frac{\mathrm{n}_2}{\mathrm{PI}}\)
⇒ \(\frac{\mathrm{n}_1}{\mathrm{PO}}+\frac{\mathrm{n}_2}{\mathrm{PI}}=\frac{\left(\mathrm{n}_2-\mathrm{n}_1\right)}{\mathrm{PC}}\) ……….. (4)
This equation shows the relation among refractive indices of media, object distance, image distance and radius of curvature.
If we take sign convention
PO = -u ; PI = v; PC = R
Substituting these values in (4),
⇒ \(\frac{\mathrm{n}_2}{\mathrm{v}}-\frac{\mathrm{n}_1}{\mathrm{u}}=\frac{\left(\mathrm{n}_2-\mathrm{n}_1\right)}{\mathrm{R}}\) …………….. (5)
It can also be used for plane surfaces.
For plane surfaces :
f = R = infinity ⇒ \(\frac{1}{\mathrm{R}}\) = 0
Then (5) ⇒ \(\frac{\mathrm{n}_2}{\mathrm{v}}-\frac{\mathrm{n}_1}{\mathrm{u}}\) = 0 ⇒ \(\frac{\mathrm{n}_2}{\mathrm{v}}-\frac{\mathrm{n}_1}{\mathrm{u}}\)
(OR)
![]()
Question 9.
Sudhakar has taken a substance in the form of wire. He applied different voltages to the wire and measured electrical currents. For this he used Ammeter and Voltmeter. He tabulated five
measurements. Then plotted a graph as shown in the figure.
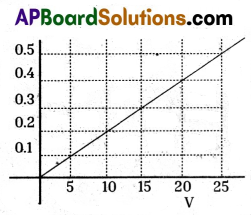
In the graph he measured voltages in volts (V) and current (I) in Amperes.
Basing on graph. Answer the following questions :
a) What type of material Sudhakar had selected for his experiment ?
b) What is the resistance of the substance ?
c) If potential difference is 20V at the ends of wire, how much electrical power is utilized by wire ?
d) What is the law associated with the above graph ?
(OR)
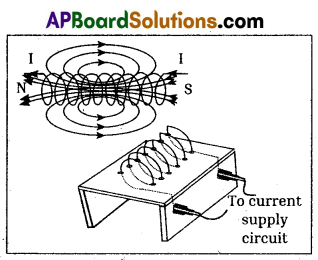
Write the experimental procedure and observations of the experiment that is to be performed to observe the magnetic field formed due to solenoid.
Answer:
a) The graph is a straight line passing through origin.
It is in the form of y = mx,
i.e., I = mV
Here, ‘m’ is slope of the graph.
Here, m = \(\frac{1}{\mathrm{R}}\) = \(\frac{1}{\text { Resistance }}\)
∴ I = (\(\frac{1}{\mathrm{R}}\))V
The substance is Ohm’s substance i.e., obeying Ohm’s law. So, it is a metal like iron spoke
(or) Copper, Aluminium etc.
b) The resistance can be known from graph is
V = IR ; R = \(\frac{V}{I}=\frac{10}{0 \times 2}=\frac{100}{2}\) = 50Ω
The reciprocal of slope of graph gives resistance.
c) The electrical power can be measured by taking the area of graph i.e., area enclosed between the straight line and X-axis.
Power (P) = Voltage × Current = VI
Area = Area of triangle = \(\frac{1}{2}\) × 4 = 4 Watts
d) Ohm’s law: The potential difference between the ends of a conductor is directly proportional to the electric current passing through it at constant temperature.
(OR)
Procedure:
- Take a wooden plank covered with white paper.
- Make holes on its surface.
- Pass copper wire through holes.
- Join the ends of the coil to a battery through switch.
- Current passes through the coil, when we switch on the circuit.
- Now sprinkle iron fillings on the surface of the plank, around the coil. Then orderly pattern f of iron fillings is seen on the paper.
- The iron fillings arrange themselves in order and look like lines of force.
- The long coil is known as solenoid. The direction of the field due to solenoid is determined by using right hand rule.
- One side of the solenoid behaves like north pole and the otherside behaves like south pole.
- Outside the solenoid, the direction of lines of force are from north to south while inside the direction is from south to north. Thus the magnetic field lines are closed loops.
- Hence electric charges in motion produce magnetic field.
Part – B (10 × 1 = 10 Marks)
Instructions :
- Answer ALL the questions.
- Each question carries 1 mark.
- In this section there are 4 options (A / B / C / D) to each question. Choose the appropriate answer and write the answer in the brackets given against the question. Part – B must be attached to the answer booklet of Part – A.
Question 1.
The mirror which has a wide field of view must be
A) concave
B) convex
C) plane
D) npne of these
Answer:
B) convex
![]()
Question 2.
A chemical equation should be balanced because the law …………… should be verified.
A) constant proportions
B) conservation of mass
C) law of equality
D) law of balance
Answer:
B) conservation of mass
Question 3.
The aqueous solution of …………….. conducts electricity.
A) Ethyl alcohol
B) Acetic acid
C) Acetone
D) Ether
Answer:
B) Acetic acid
Question 4.
The reaction of an acid with a base to give a salt and water is known as
A) Neutralization
B) Reduction
C) Oxidation
D) Crystallisation
Answer:
A) Neutralization
Question 5.
Which of the following aqueous solution is called brine ?
A) sodium chloride
B) potassium chloride
C) copper chloride
D) calcium chloride
Answer:
A) sodium chloride
Question 6.
A person is standing on the bank of a river. A fish inside water will see the person to be
A) taller
B) shorter
C) original height
D) depends on fish
Answer:
A) taller
Question 7.
The minimum distance between an object and its real image formed by a convex lens is
A) \(\frac{2}{3}\)f
B) 2f
C) \(\frac{5}{2}\)f
D) 4f
Answer:
A) \(\frac{2}{3}\)f
![]()
Question 8.
The persistence if vision ofr normal eye is
A) (\(\frac{1}{18}\))th of a second
B) (\(\frac{1}{6}\))th of a second
C) (\(\frac{1}{10}\))th of a second
D) (\(\frac{1}{16}\))th of a second
Answer:
D) (\(\frac{1}{16}\))th of a second
Question 9.
Which of the following element belongs to 3 period and also a 17 (VILA) group?
A) Fluorine
B) Bromine
C) Oxygen
D) Chlorine
Answer:
D) Chlorine
Question 10.
Which of the following is the outlet through which fuel gases go out the furnace ?
A) Chimney
B) Flux
C) Hearth
D) Fire box
Answer:
A) Chimney