Access to a variety of AP Inter 2nd Year Chemistry Model Papers and AP Inter 2nd Year Chemistry Question Paper May 2019 helps students overcome exam anxiety by fostering familiarity. question patterns.
AP Inter 2nd Year Chemistry Question Paper May 2019
Note : Read the following instructions carefully.
- Answer all questions of Section – ‘A’. Answer any six questions in Section – ‘B’ and any two questions in Section – ‘C’.
- In Section – A, questions from Sr. Nos. 1 to 10 are of “Very Short Answer Type”. Each question carries two marks. Every answer may be limited to 2 or 3 sentences. Answer all these questions at one place in the same order.
- In Section -”’8′, questions from Sr. Nos. 11 to 18 are of “Short Answer Type”. Each question carries four marks. Every answer may be limited to 75 words.
- In Section – ‘C’, questions from Sr. Nos. 19 to 21 are of “Long Answer Type”. Each question carries eight marks. Every answer may be limited to 300 words.
- Draw labelled diagrams wherever necessary for questions in Section – ‘B’ and ‘C’.
Section – A
Note : Answer all the questions.
Question 1.
What is an Ideal Solution?
Answer:
A solution of two or more components which obeys Raoult’s law at all concentrations and at all temperatures is called ideal solution. In ideal solution there should not be any association between solute and solvent, (i.e.) no chemical interaction between solute and solvent of solution.
Ex : The following mixtures form ideal solutions.
- Benzene + Toluene
- n – hexane + n – heptane
- ethyl bromide + ethyl iodide
Question 2.
Write the Arrhenius equation for the rate constant (K) of a reaction.
Answer:
Arrhenius equation is
k = A × e-Ea/RT
k = Rate constant
Ea = activation energy
R = gas constant
T = Temperature (K)
![]()
Question 3.
Give the composition of the following alloys :
(a) Brass
(b) Bronze
Answer:
a) Composition of Brass : 60 – 80% Cu, 20 – 40% Zn
b) Composition of Bronze : 75 – 90% Cu, 10 – 25% Sn.
Question 4.
How is XeOF4 prepared ? Give its structure.
Answer:
Partial hydrolysis of XeFg gives XeOF4
XeF6 + H20 → XeOF4 + 2HF
XeOF4 is a colourless volatile liquid it has a square pyramidal shape.
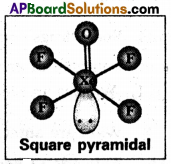
Question 5.
Write the reactions of F2 and Cl2 with water.
Answer:
Fluorine produces 02 and 03 on passing through water.
3 F2 + 3 H2O → 6 HF + O3
2 F2 + 2 H2O → 4 HF + O2
Chlorine dissolves in water giving a solution of chlorine water. A freshly prepared solution of chlorine water contains HCl and HOCl.
Cl2 + H2O → HCl + HOCl
HOCl is unstable and dissociates to give nascent oxygen
HOCl → HCl + (O)
Question 6.
Scandium is a transition element. But Zinc is not. Why ?
Answer:
Scandium has electronic configuration [Ar] 4s23d1.
Zinc has electronic configuration [Ar] 4s23d10.
Scandium has one unpaired d-electron where as Zinc has zero unpaired d-electrons so Scandium is transition element but Zinc is not.
Question 7.
What is PDI (Poly Dispersity Index)?
Answer:
Poly Dispersity Index (PDI) : The ratio between weight average molecular mass (Μw) and the number average molecular mass (Μn) of a polymer is called Poly Dispersity Index (PDI).
Question 8.
What is allosteric site?
Answer:
Some drugs do not bind to the enzyme’s active site and bind to a different site of enzyme. This different site of enzyme is called allosteric site.
Question 9.
What are antacids? Give exampl1e.
Answer:
Antacids : Chemicals that remove the excess of acid in the stomach and maintain the pH to normal level are antacids. E.g. : Omeprozole, Lansoprazole etc.,
![]()
Question 10.
Write the names and structures of the monomers used for getting the following :
a) Poly Vinyl Chloride
b) Teflon
Answer:
i) Polyvinyl chloride
Monomer : Vinyl chloride
Structure : CH2 = CH – Cl
ii) Teflon
Monomer : Tetrafluoro ethylene
Structure : CF2 = CF2
Section – B
Question 11.
Derive Bragg’s equation.
Answer:
Derivation of Bragg’s equation : When X-rays are incident on the crystal or plane, they are diffracted from the lattice points (lattice points may be atoms or ions or molecules). In the crystal the lattice points are arranged in regular pattern. When the waves are diffracted from these points, the waves may be constructive or destructive interference.
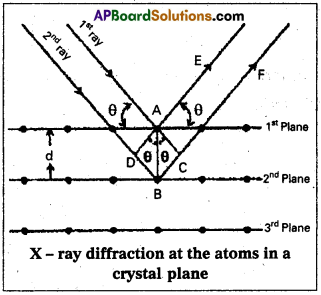
The 1st and 2nd waves reach the crystal surface. They undergo constructive interference.Then from the figure 1st and 2nd rays are parallel waves. So, they travel the same distance till the wave form AD. The second ray travels more than the first by an extra distance
(DB + BQ after crossing the grating for it to interfere with the first ray in a constructive manner. Then only they can be in the same phase with one another. If the two waves are to be in phase, the path difference between the two ways must be equal to the wavelength (λ) or integral multiple of it (n λ, where n = 1, 2, 3,).
(i.e.,) nλ = (DB + BC) [where n = order of diffraction]
DB = BC = d sin θ
θ = angle of incident beam,
(DB + BC) = 2d sin θ
d = distance between the planes
nλ = 2d sin θ
This relation is known as Bragg’s equation.
Question 12.
Define Mole fraction. Calculate the Mole fraction of ethylene glycol (C2H6O2) in a solution containing 20% of C2H6O2 by mass.
Answer:
Mole fraction : The ratio of number of moles of the one component of the solution to the total number of moles of all the components of the solution is called mole fraction.
Mole fraction of solute Xs = \(\frac{n_s}{n_0+n_s^{\prime}}\)
Mole fraction of solute X0 = \(\frac{n_s}{n_0+n_s^{\prime}}\)
ns = number of moles of solute
n0 = number of moles of solute solvent
It has no units
Calculate the Mole fraction of ethylene glycol (C2H6O2) in a solution containing 20% of C2H6O2 by mass : Assume that we have lOOg of solution (one can start with any amount of solution because the results obtained will be the same). Solution will contain 20g of ethylene glycol and 80g of water.
Molar mass of C2H6O2 = 12 × 2 + 1 × 6 + 16 × 2 = 62 g mol-1
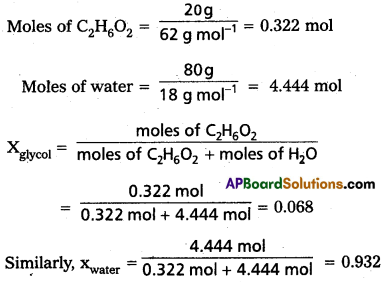
Mole fraction of water can be calculated as : 1 – 0.068 = 0.932
Question 13.
What is an Emulsion? How emulsions are classified. Give one example for each type of emulsion.
Answer:
Emulsion : The colloidal system in which a dispersion of finely divided droplets of a liquid in another liquid medium is called emulsion. Eg : Milk, Vanishing cream, Cold cream.
Emulsion : A dispersion of finely divided droplets of a liquid in another liquid medium is called ’emulsion’.
Ex : Milk.
In Milk, the droplets of liquid fat are dispersed in water. This is an example for oil in water type emulsion. Classification of emulsions : Emulsions are classified into two classes. These are
a) oil in water (O/W) and
b) water in oil (W/O), (O = Oil; W = Water).
These emulsions are classified as such depending on which is dispersed phase and which is dispersion medium.
a) Oil in Water (O/W) type emulsions : In this type of emulsions, the dispersed phase is oil and the dispersion medium is water. Ex : Milk, liquid, fat (oil) in water. Vanishing cream; fat in water.
b) Water in Oil (W/O) type emulsions : In this type of emulsions, the dispersed phase is water and the dispersion medium is oil.
Ex : Stiff greases : water in lubrication oils
Cod liver oil : water in cod liver oil
Cold cream : water in fat.
![]()
Question 14.
Explain the following :
a) Zone refining
b) Poling .
Answer:
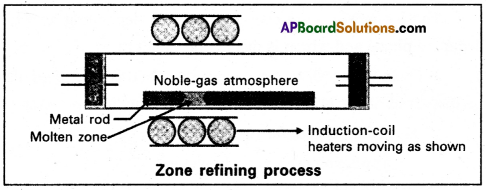
a) Zone refining :
- Zone refining is based on the principle that the impurities are more soluble in the melt than in the solid state of the metal.
- A circular mobile heater is fixed at one end of a rod of impure metal.
- The molten zone moves along with the heater moves forward the pure metal crystallises out of the melt and the impurities pass into the adjacent molten zone.
- The above process is repeated several times and the heater is moved in the same direction form one end to the other end. At one end impurities get concentrated. This end is cut off.
- This method is very useful for producing semiconductor grade metals of very high purity. Eg: Ge, Si,B,Ga ets….
b) Poling :
When the metals are having the metal oxides as impurities this method is employed. The impure metal is melted and is then covered by carbon powder. Then it is stirred with green wood poles. The reducing gases formed from the green wood and the carbon, reduce the oxides to the metal. Eg: Cu & Sn metals are refined by this method.
Question 15.
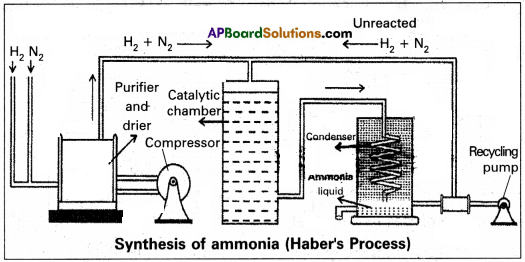
How is ammonia manufactured by Haber’s process?
Answer:
In Haber process ammonia is directly synthesised from elements (nitrogen and hydrogen).
The principle involved in this is
N2 (g) + 3H2 (g) ⇌ 2NH3 + 92. kj
This is a reversible exothermic reaction
According to Le Chatelier’s principle favourable conditions for the better yield of ammonia are low temperature and high pressure. But the optimum conditions are :
Temperature : 720k
Pressure : 200 atmospheres
Catalyst : Finely divided iron in the presence of molybdenum (Promoter).
Procedure : A mixture of nitrogen and hydrogen in the volume ratio 1 : 3 is heated to 725 – 775K at a pressure of 200 atmospheres is passed over hot finely divided iron mixed with small amount of molybdenum as promotor. The gases coming out of the catalyst chamber consists of 10 – 20% ammonia gas are cooled and compressed, so that ammonia gas is liquified, and the uncondensed gases are sent for recirculation.
![]()
Question 16.
Explain geometrical isomerism in co-ordination compounds giving suitable examples.
Answer:
1) Geometrical isomerism arises in Co-ordination complexes due to different possible geometric arrangements of the ligands.
2) This isomerism found in complexes with Co-ordination numbers 4 and 6.
3) In a square planar complex of formula [MX2L2] the two ligands may be arranged in adjacent to each other in a cis isomer (or) opposite to each other in a trans isomer.
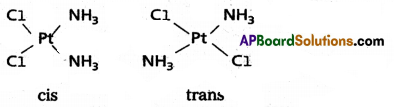
4) Geometrical isomers (cis and trans) of [Co(NH3)3Cl2] uare planar complex of type [MAB XL] shows three isomers two-cis and one trAnswer: (A, B, X, L are unidentate ligands is square planar complex).
5) Geometrical isomerism is not possible in tetrahedral geometry.
6) In octahedral complexes of formula [MX2L4] in which two ligands X may be oriented cis or trans to each other.
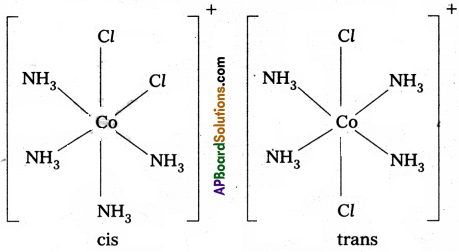
Geometrical isomers (cis and trans) of Pt[(NH3)4Cl2]+
Another type of geometrical isomerism occurs in octahedral Co-ordination compounds of type [Ma3b3] if three donar atoms of the same ligands occupy adjacent positions at the comers of an octahedral face then it is facial (fac) isomer. When the positions occupied are around the meridian of the octahedran then it is meridonial (mer) isomer.
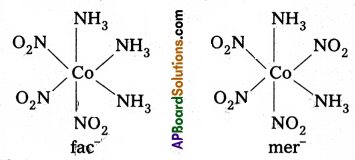
The facial (fac) and meridonial (mer) isomers of [CO(NH3)3(NO2)3]
Question 17.
Give the sources of the following vitamins and name the disease caused by their dificiency.
(a) A
(b) D
(c) E
(d) K
Answer:
| Vitamin | Sources | Deficiency diseases |
| A | Fish oils, liver, kidney | Night blindness, Redness in eyes. |
| D | Fish oils, butter, milk, egg | Rickets in children,osteomalacia in adults |
| E | Wheat germ oil, egg yolk, green vegetables | Sterility |
| K | Green vegetables, Intestinal flora. | Blood coagulation is prevented |
Question 18.
Explain the Grignard reagents preparation and application with suitable example.
Answer:
Alkyl magnesium halides are generally called as Grignard reagenty.
Preparation : These are prepared by the treatment of alkyl halides with magnesium metal in presence of dry ether.
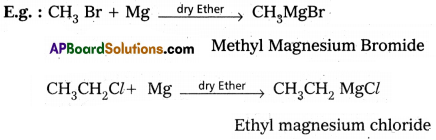
Applications :
Grignard reagents have wide applications in the synthesis of large no. of organic compounds.
1) Preparation of alkanes :
Grignard reagents reacts with alcohols and forces alkanes.
CH3 Mg Br + C2H5OH → CH4 + C2H5O MgBr
CH3 CH2 MgCl + C2H5OH → C2H6 + C2H5O MgBr
2) Preparation of alcohols :
Ethylalcohol is obtained by the action of Methyl magnesium bromide on formal dehyde followed by the hydrolysis.
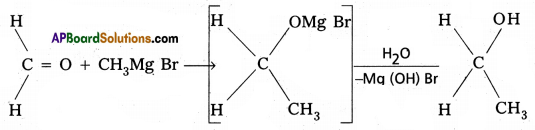
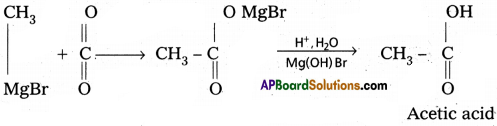
3) Preparation of carboxylicacids :
Grignard reagent on carboxylation followed by the hydrolysis to form carboxylic acids.
Question 19.
(a) State and explain Kohlrausch’s law of independent migration of ions.
Answer:
Kohlrausch’s lafw of independent migration of ions : The limiting molar conductivity of an electrolytes can be represented as the sum of the individual contributions of the anion and the cation of the electrolytes.
λm(AB)0 = λA+0 + λ B–0
λm0 = Limiting molar conductivity
λA+0 = Limiting molar conductivity of cation
λB–0 = Limiting molar conductivity of anion
Applications :
1) Kohlrausch’s law is used in the calculation of the limiting molar conductivity of weak electrolytes.
Eg : λCH3COOH = λCH3COONa + λHCl – λNaCl
= λCH3COO– + λna+ + λH+ + λCl– – λNa+ – λCl–
= λCH3COO– + λH+
2) This law is used in the calculation of degree of dissociation – of a weak electrolyte.
3) This law is used in the calculation of solubility of sparingly sqluble salts like AgCl, BaSQ4 etc.
(b) What is “molecularity” of a reaction ? How is it different from the “order” of a reaction ? Name one bimolecular and one trimolecular gaseous reactions.
Answer:
The number of reacting species (atoms, ions or molecules) taking parts in an elementary reaction, which must colloid simultaneously to bring about a chemical reaction is called molecularity of a reaction.
NH4NO2 → N2 + 2H2O (Unimolecular)
2HI → H2 + I2 (Bimolecular)
2NO + O2 → 2NO2 ( Trimolecular)
Molecularity has only integer values (1, 2, 3 ………) It has non zero, non, fraction values while order has zero, 1, 2, 3 and fractional values. It is determined by reaction mechanism, order is determined experimentally.
![]()
Question 20.
How is ozone prepared from oxygen ? Explain its reaction with
(a)C2H4
(b) KI
(c) Hg
(d) PbS
Answer:
Preparation of Ozone :
A slow dry stream of oxygen under silent electric discharge to form ozone (about 10%). The product obtained is known as ozonised oxygen.
3O2 → 2O3 ∆H° = 142kJ/mole
The formation of ozone is an endothermic reaction. It is necessary to use silent electric discharge in the preparation of Os to prevent its de composition
a) Reaction with C2H4 : Ethylene reacts with ozone to form Ethylene ozonoid followed by the hydrolysis to form formaldehyde.
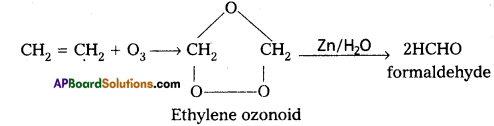
b) Reaction with KI: moist KI is oxidised to Iodine in presence of ozone.
2KI + H2O + O3 → 2KOH + I2 + O2
c) Reaction with Hg :
Mercury loses it’s lustreness, meniscus and consequently sticks to the walls of glass vessel when it reacts with ozone. This phenomenon is called tailing of mercury.
2Hg + O3 → Hg2O + O2
It is removed by. shaking it with water which dissolves Hg2O.
d) Reaction with PbS : Black lead sulphide oxidised to white lead sulphate in presence of ozone.
PbS + 4O3 → PbSO4 + 4O2.
Question 21.
a) Describe the following :
(i) Cannizaro reaction
(ii) Decarboxylation
Answer:
i) Cannizaro reaction : On treating with concentrated alkali, aldehydes which do not have any a – hydrogen atom, urtdergo self oxidation and reduction (disproportionation) reaction. This reaction is called cannizaro reaction.
As a result, one molecule of aldehyde is reduced to alcohol while another is oxidised to carboxylic acid salt. For example
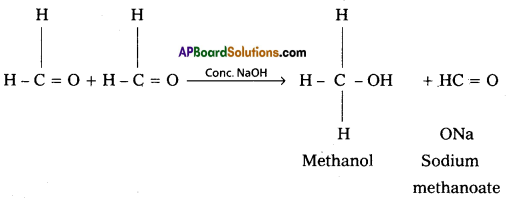
ii) Decarboxylation
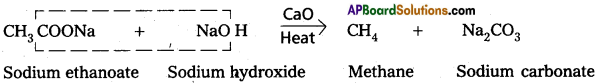
Carboxylic acids lose carbon dioxide molecule to produce hydrocarbons on heating their sodium salts with sodalime (a mixutre of NaOH & CaO in ratio 3:1). This reaction is called decarboxylation
(b) How do you prepare Ethyl cyanide and Ethyl isocyanide from a common alkyl halide ?
Answer:
Preparation of ethyl cyanide :
Ethyl chloride reacts with aq. Ethanolic KCN to form Ethyl cyanide as a major product
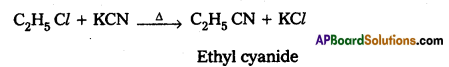
Preparation of Ethyl isocyanide :
Ethyl chloride reacts with aq. Ethanolic AgCN to form Ethyl iso cyanide as a major product
C2H5 Cl + AgCN → C2H5 NC + AgCl Ethyl isocyanide