Access to a variety of AP Inter 2nd Year Chemistry Model Papers and AP Inter 2nd Year Chemistry Question Paper March 2019 helps students overcome exam anxiety by fostering familiarity. question patterns.
AP Inter 2nd Year Chemistry Question Paper March 2019
Note : Read the following instructions carefully.
- Answer all questions of Section – ‘A’. Answer any six questions in
Section – ‘B’ and any two questions in Section – ‘C’. - In Section – A, questions from Sr. Nos. 1 to 10 are of “Very Short Answer Type”. Each question carries two marks. Every answer may be limited to 2 or 3 sentences. Answer all these questions at one place in the same order.
- In Section -”’8′, questions from Sr. Nos. 11 to 18 are of “Short Answer Type”. Each question carries four marks. Every answer may be limited to 75 words.
- In Section – ‘C’, questions from Sr. Nos. 19 to 21 are of “Long Answer Type”. Each question carries eight marks. Every answer may be limited to 300 words.
- Draw labelled diagrams wherever necessary for questions in Section – ‘B’ and ‘C’.
Section – A
Note : Answer all the questions.
Question 1.
What is relative lowering of vapour pressure?
Answer:
Relating lowering of vapour pressure : The ratio of lowering of vapour pressure of a solution containing non-volatile solute to the vapour pressure of pure solvent is called relative lowering of vapour pressure.
R.L.V.P. = \(\frac{P_0-P_S}{P_0}\)
po – ps = lowering of vapour pressure
po = Vapour pressure of pure solvent
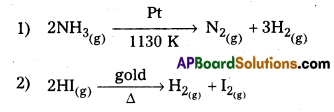
Question 2.
Give two examples for zero order reaction.
Answer:
Examples for zero order reactions Pt
Question 3.
Give the composition of the following alloys.
a) Brass
b) Bronze
c) German Silver
Answer:
a) Composition of Brass : 60 – 80% Cu, 20 – 40% Zn
b) Composition of Bronze : 75 – 90% Cu, 10 – 25% Sn
c) Composition of German silver : 50 – 60% Cu, 10 – 30% Ni, 20 – 30% Zn.
![]()
Question 4.
What happens when white phosphorus is heated with cone. NaOH solution in an inert atmosphere of CO2?
Answer:
When white phosphorus heated with con. NaOH solution in an inert atmosphere of 002 forms PH3. .
P4 + 3NaOH + 3H2O — PH3 + 3NaH2PO2.
Question 5.
How is chlorine manufactured by Deacon’s method?
Answer:
Deacon’s process : In Deacon’s process chlorine is obtained by the oxidation of hydrogen chloride gas by atmospheric oxygen in the presence of CuCl2 (catalyst) at 723 K.
![]()
Question 6.
What is Misch metal ? Give its composition and use.
Answer:
Mischmetall is an alloy which consists of a lanthanoid metal (~ 95%) and iron (~ 5%) and traces of S, C, Ca and Al.
It is used in Mg- based alloy to produce bullets, shell and lighter flint.
Question 7.
What is PHBV ? How is it useful to man?
Answer:
Poly β – hydroxy butyrate – CO – β – hydroxy Valerate (PHBV) : It is a Copolymer of 3 -hydroxy butanoic acid and 3 – hydroxy pentanoic acid.
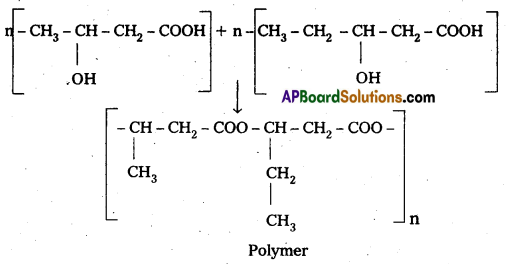
Properties & Uses :
The properties of PHBV vary according to the ratio of both the acids, 3- hydroxy butanoic acid provides stiffness and 3-hydroxy pentanoic acid imparts flexibility to copolymer.
It is used in medicine for making capsules. PHBV also undergoes degradation by bacteria.
Question 8.
What is PDI (Poly Dispersity Index)?
Answer:
Poly Dispersity Index (PDI): The ratio between weight average molecular mass (Mw) and the number average molecular mass (Mn) of a polymer is called Poly Dispersity Index (PDI).
Question 9.
What are analgesics ? How are they classified?
Answer:
Analgesics: These are to reduce or totally abolish pain without causing impairment of consciousness, mental confusion, in coordination, paralysis, disturbances of nervous system etc.
Analgesics are classified as :
i) Narcotic analgesics :
These are most potent and clinically useful agents causing depression of central nervous system and at the same time act as strong analgesics. E.g. : Morphine, Codeine etc.
ii) Non-narcotic analgesics :
These drugs are analgesics but they have no addictive properties. Their analgesic use is limited to mild aches and pains. E.g. : Aspirin, Ibuprofen etc.
Question 10.
What are antiseptics ? Give examples.
Answer:
Antiseptics : The chemical compounds that kill (or) prevent the growth of micro organism are called antiseptics. Antiseptics are the chemical substances applied on the living tissues such as wounds, cuts, ulcers and diseased skin surfaces. E.g.: Dettol, Bithional etc.
Section – B
Question 11.
Describe the two main types of semi-conductors and contrast their conduction mechanism.
Answer:
The solids which are having moderate conductivity between insulators and conductors are called semi conductors.
1) These have the conductivity range from 10-6 to 104 Ohm-1
2) By doping process the conductivity of semi conductors increases. E.g. : Si, Ge, crystal. Semi conductors are of two types. They are :
1. Intrinsic semi – conductors :
In case of semi – conductors, the gap between the valence band and conduction band is small. Therefore, some electrons may jump to conduction band and show some conductivity. Electrical conductivity of semi- conductors increases with rise in “temperature”, since more electrons can jump to the conduction band. Substances like silicon and germanium show this type of behaviour and are called intrinsic semi – conductors.
2. Extrinsic semi – conductors :
Their conductivity is due to the presence of impurities. They are formed by “doping”.
Doping : Conductivity of semi-conductors is too low to be of practical use. Their conductivity is increased by adding an appropriate amount of suitable impurity. This process is called “doping”.
Doping can be done with an impurity which is electron rich or electron deficient. Extrinsic semi-conductors are of two types,
a) n-type semi-conductors :
It is obtained by adding trace amount of V group element (P, As, Sb) to pure Si or Ge by doping.
When P, As, Sb (or) Bi is added to Si or Ge some of the Si or Ge in the crystal are replaced by P or As atoms and four out of five electrons of P or As atom will be used for bonding with Si or Ge atoms while the fifth electron serve to conduct electricity.
b) p-type semi-conductors :
It is obtained by doping with impurity atoms containing less electrons i.e., Ill group elements (B, AZ, Ga or In). When B or AZ is added to pure Si or Ge some of the Si or Ge in the ciystal are replaced by B or AZ atoms and four out of three electrons of B or AZ atom will be used for bonding with “Si” or Ge atoms while the fourth valence electron is missing is called electron hole (or) electron vacancy. This vacancy on an atom in the structure migrates from one atom to another. Hence it facilitates the electrical conductivity.
Question 12.
Vapour pressure of water at 293K is 17.535 mm Hg. Calculate the vapour pressure of the solution at 293K when 25g of glucose is dissolved in 450g of water.
Answer:
Raoult’s law formula
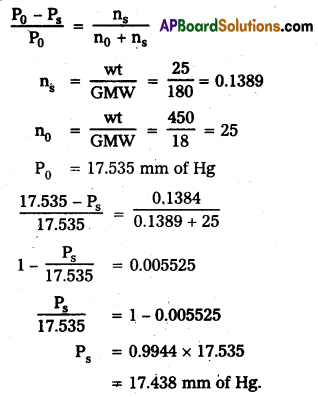
Question 13.
Name any four enzyme catalysed reactions.
Answer:
i) Inversion of Cane sugar : Enzyme : Invertase ‘ Invertase

ii) Conversion of glucose into Enthyl alcohol :
Enzyme : Zymase
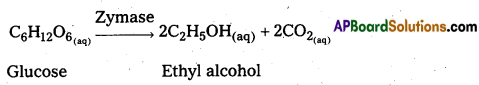
iii) Conversion of starch into maltose : Enzyme : Diastase
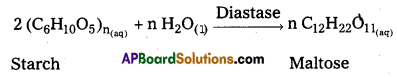
iv) Conversion of maltose into glucose : Enzyme : Maltase
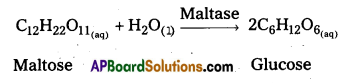
v) Decomposition of urea into ammonia : Enzyme : Urease
![]()
Vi) Conversion of milk to curd : Enzyme : Lacto bacilli Milk lacto bacilli;
![]()
Question 14.
Explain the purification of Sulphide ore by Froth Floatation
method.
Answer:
Froth floatation method :
- This method is used to concentrate sulphide ores.
- In this process a suspension of the powdered ore is made with water.
- A rotating paddle is used to agitate the suspension and air is blown into the suspension in presence of an oil.
- Froth is formed as a result of blown of air, which carries the mineral particles.
- To the above slurry froth collectors and stabilizers are added.
- Collectors like pine oil enhance non-wettability of the mineral particles.
- Froth stabilizers like cresol stabilize the froth.
- The mineral particles wet by oil and gangue particles wet by water.
- The froth is light and is skimmed off. The ore particles are then obtaiined from the froth
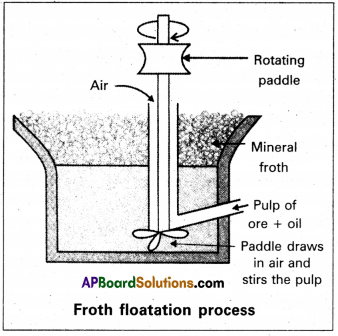
By using depressants in froth floatation process, it is possible to separate a mixture of two sulphide ores. Eg : In the ore containing ZnS and PbS, the depressant used is NaCN. It prevents ZnS from coming to the froth but allows PbS to come with the froth.
Question 15.
How are XeF2 and XeF4 prepared? Give their structures.
Answer:
Xenon forms the binary fluorides XeF2, XeF4 as follows. These are formed by direct combination of Xe and F2.
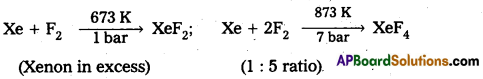
Structure of XeF2 :
1) XeF2 central atom is Xe’.
2) ‘Xe’ undergoes sp3d hybridisation in it’s 1stst excited state.
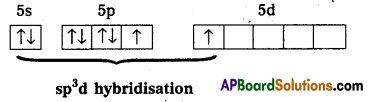
3) Shape of molecule is linear.
4) Xe form two a – bonds with two fluorines, (sp3 – 2pz overlap)
b) Structure of XeF4:
1) Central atom in XeF4 is ‘Xe’.
2) Xe undergoes sp3d2 hybridisation in it’s 2nd excited, state.
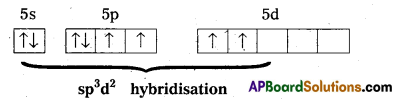
3) Shape of the molecule is square planar with bond angle 90° and bond length 1.95A.
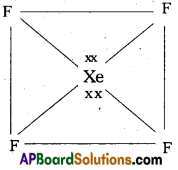
4) Xe – forms four o – bonds by the overlap of sp3d2 – 2pZ(F) orbitals.
Question 16.
Using IUPAC names. Write the systematic names of the following.
(i) [Co(NH3)6]Cl3
(ii) [Fe(C2O4)3]-4
(iii) [Fe(CN)6]-4
(iv) [NiCl4]-2
Answer:
i) Co(NH3)6]Cl3 – Hexa amine cobalt (III) chloride
ii) [Fe(C2O4)3]-4 – Trisoxalato ferrate (II) ion
iii) [Fe(CN)6]-4 – Hexa cyano ferrate (II) ion
iv) [NiCl4]-2 – Tetra chlbro Nickelate (II) ion
![]()
Question 17.
What are hormoties ? Give orie exaftiple for’each
i) Steroid Hormones
ii) Polypeptide Hormones
iii) Amino acid derivatives.
Answer:
Hormones : Hormone is defined as an “organic compound synthesised by the ductless glands of the body and carried by the blood stream to another part of the body for its function”.
Eg : testosterone, estrogen.
- Example for steroid hormones : Testosterone, Estrogen
- Example for Polypeptide Hormones : Insulin
- Example for Amino acid derivative: Thyroidal hormones thyroxine.
Question 18.
Which compound in each of the following pairs will react faster in SN2 reaction with OH-2?
i) CH3Br (Or) CH3I
ii) (CH3)3 CCl (or) CH3Cl
Answer:
i) Among CH3Br and CH3I, CH3 -1 reacts faster in SN2 reaction with OHθ because bond dissociation energy of C – I is less than the bond dissociation energy of C – Br.
ii) Among CH3Cl and (CH3)3CCl, CH3 – Cl reacts faster in SN2 reaction with OHθ because (CH3)3CCl has high steric hindrance than CH3Cl.
Section – C
Question 19.
Give the different types of Batteries and explain the constru* ction and working of each type of battery.
Answer:
The batteries which after their use over a period of time, becomes dead and the cell reaction is completed and this cannot be reused again are called primary batteries. Eg.: Leclanche cell, dry cell.
Secondary battery:
A secondary battery is the battery in which after it’s use can be recharged and can be used again. A good secondary battery undergoes large no. of discharging and charging cycles. Lead storage battery is an example of secondary battery. The cell reactions when the battery is in use are
Pb(s) + SO4-2(aq) → PbSO4(s) + 2e– (Anode)
PbO2(S) + SO4-2(aq) > 4H+(aq)(aq) + 2e– → PbSO4(s) + 2H2Ol (Cathode)
Overall cell reaction is
Pb(s) + PbO2(s) + 2H2SO4(aq) → 2PbSO4(s) + 2H2O(l)
Construction and working of primary batteries :
Dry cell :
1. This is a modification of Leclanche cell. The liquid state electrolytes (in Leclanche cell) are replaced by paste electrolytes.
2. A cylindrical Zn’vessel is covered with a cardboard. This is sealed with pitch. Zn vessel acts as negative electrode. A carbon rod is introduced at the centre of the Zn vessel. This carbon rod acts as positive electrode.
3. Carbon rod is surrounded by a paste of (C+ MnO2). The remaining space is filled with (NH4Cl + ZnCl2) paste. The two pastes are separated by a porous sheet.
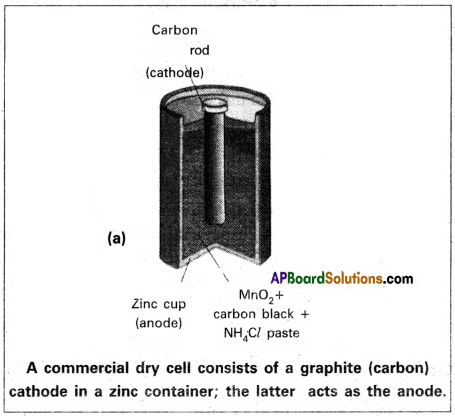
4. These cells are easy to handle and used in radios, watches, torch lights etc. The cell potential’is approximately 1.5 V.
5. Electrode reactions :
Cathode : MnO2 + NH4+ + eθ → MnO (OH) + NH3
Anode : Zn + 2MnO2 + 2H2O → Zn2+ + 2OHθ + 2MnO (OH)
Some of the secondary reactions are
2 NH4Cl + 2OHθ → 2NH3 + 2Cl– + 2H2O
Zn2+ + 2NH3 + 2Cl– → [Zn (NH3)2] Cl2
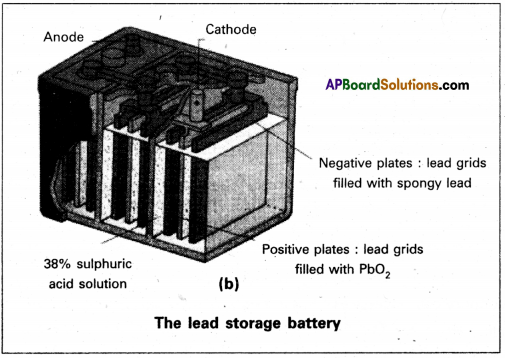
Secondary batteries – construction and working:
A secondary cell after its use can be recharged and can be used again. A good secondary cell undergoes a large number of discharging and charging cycles. The most important secondary cell in use is the lead storage battery (fig. (b)). This is commonly used in automobiles and invertors. It consists of a lead anode and a grid of lead packed with lead dioxide (PbO^ as cathode. A 38% solution of sulphuric acid is used as electrolyte.
The cell reactions when the battery is in use (discharging) are :
Anode : Pb(s) + SO42- → PbSO4(s) + 2e–
Cathode : PbO2(s)+ SO4(aq)2- + 4H+(aq) + 2e– → pbSO4(s) + H2O(l)
i.e., overall cell reaction consisting of cathode and anode reactions is
Pb(s) + PbO2(s) + 2H2SO4(aq) → 2 PbSO4(s) + 2H2O(l)
These reactions occur during discharge i.e., during use of the battery.
On charging the discharged battery the above reaction is reversed and PbSO4(s) on anode and cathode is converted into Pb and PbO2, respectively.
Question 20.
How is ozone prepared from oxygen ? Explain its reaction with
i) C2H4
ii) KI
iii) Hg
iv) PbS
Answer:
Preparation of Ozone :
A slow dry stream of oxygen under silent electric discharge to form ozone (about 10%). The product obtained is known as ozonised oxygen.
3O2 → 2O3 ΔH° = 142kJ/mole
- The formation of ozone is an endothermic reaction.
- It is necessary to use silent electric discharge in the preparation of O3 to prevent its de composition
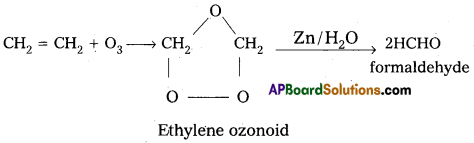
a) Reaction with C2H4 : Ethylene reacts with ozone to form Ethylene ozonoid followed by the hydrolysis to form formal dehyde.
b) Reaction with KI: moist KI is oxidised to iodine in presence of ozone.
2KI + H2O + O3 → 2KOH + I2 + O2
c) Reaction with Hg:
Mercury loses it’s lustreness, meniscus and consequently sticks to the walls of glass vessel when it reacts with ozone. This phenomenon is called tailing of mercury.
2Hg + O3 → Hg2O + O2
It is removed by shaking it with water which dissolves Hg2O.
d) Reaction with PbS : Black lead sulphide oxidised to white lead sulphate in presence of ozone.
PbS + 4O3 → PbSO4 + 4O2.
![]()
Question 21.
Describe the following :
i) Acetylation
ii) Cannizaro reaction
iii) Cross Aldol condensation
iv) Decarboxylation.
Answer:
i) Acetylation:
When active hydrogen atom of alcohol, phenol (or) an amine is replaced by acetyl (CH3CO-) group to form corresponding ester (or) amide, the reaction is known as acetylation. Reagents used are acid chloride (or) acid anhydride in presence of a base like pyridine (or) dimethylaniline. For exmple:
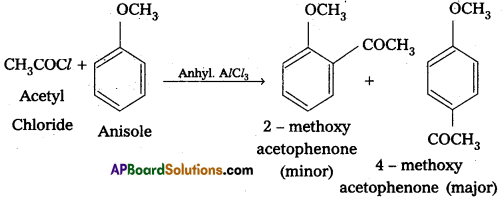
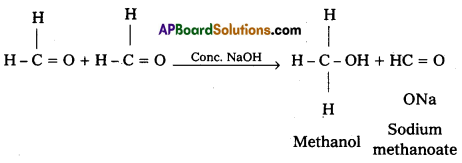
ii) Cannizaro reaction :
On treating with concentrated alkali, aldehydes which do not have any α – hydrogen atom, undergo self oxidation and reduction (disproportionation) reaction. This reaction is called cannizaro reaction. As a result, one molecule of aldehyde is reduced to alcohol while another is oxidised to carboxylic acid salt.
iii) Cross aldol condensation : When aldol condensation is carried out between two different aldehydes and (or) ketones, it is called cross aldol condensation. If both the reactants contain α – hydrogen atoms, it gives a mixture of four products.
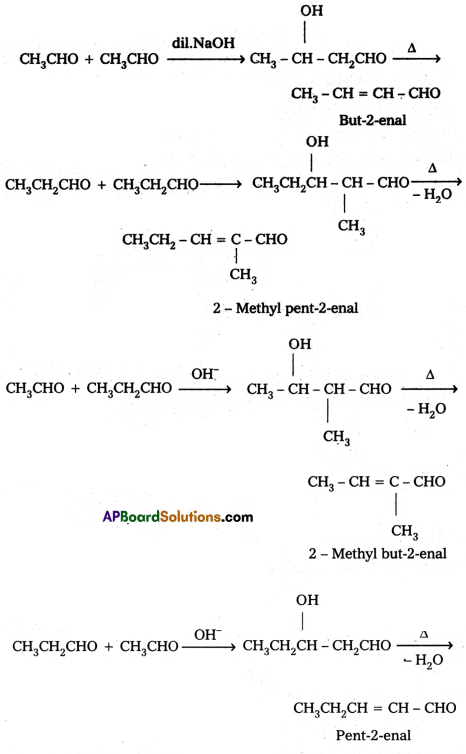
Ketones can also be used as one component in the cross aldol reactions
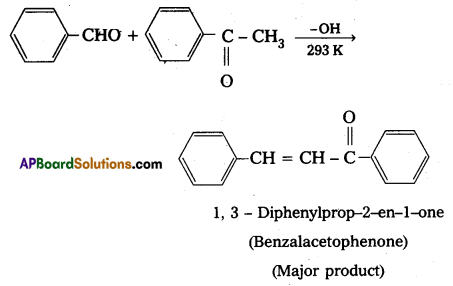
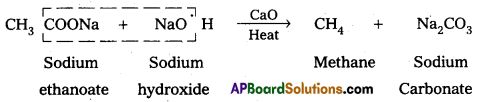
iv) Decarboxylation :
Carboxylic acids lose carbon dioxide molecule to produce hydrocarbons on heating their sodium salts with sodalime (a mixture of NaOH & CaO in ratio 3:1). This reaction is called decarboxylation.