Students get through AP Inter 1st Year Physics Important Questions 13th Lesson Thermodynamics which are most likely to be asked in the exam.
AP Inter 1st Year Physics Important Questions 13th Lesson Thermodynamics
Very Short Answer Questions
Question 1.
Define Thermal equilibrium. How does it lead to Zeroth law’ of Thermodynamics? [Imp.Q]
Answer:
Thermal equilibrium:
Two systems are said to be in thermal equilibrium, if the temperatures of two systems are equal and there is no net flow of heat between them when they are brought into thermal contact.
Zeroth law of Thermodynamics:
If two systems are in thermal equilibrium with a third system separately then they must be in thermal equilibrium with each other.
Question 2.
Define Calorie. What is the relation between calorie and mechanical equivalent of heat? Ilmp.Qi
Answer:
Calorie :
The amount of heat required to raise the temperature of one gram of water through 1°C at a pressure of 1 atm, is called calorie.
Mechanical equivalent of heat (J) is defined as the amount of work needed to produce 1 calorie heat.
Therefore 1 calorie = 4.186 joule
Question 3.
What thermodynamic variables can be defined by (a) Zeroth law (b) First law? [Imp.Q]
Answer:
a) Thermodynamic variable defined by Zeroth law of thermodynamics is temperature.
b) Thermodynamic variable defined by First law of thermodynamics is internal energy.
Question 4.
Define specific heat capacity of the substance. On what factors does it depend? [Imp.Q]
Answer:
Specific heat capacity :
The amount of heat required by unit mass of substance to raise its temperature by one degree is called specific heat capacity.
S = \(\frac{1}{m}\)(\(\frac{dQ}{dT}\))
Specific heat capacity depends on the nature of the substance and its temperature.
Question 5.
Define molar-specific heat capacity. [Imp.Q][May 13]
Answer:
Molar-specific heat capacity :
The amount of heat required by one mole of substance to raise its temperature by one degree is called molar-specific heat capacity.
C = \(\frac{1}{n}\)(\(\frac{dQ}{dT}\))
SI units : Jmol-1K-1
Molar-specific heat capacity depends on the nature of the substance, its temperature and the conditions under which heat is supplied.
![]()
Question 6.
For a solid, what is the total energy of an oscillator? [Imp.Q]
Answer:
Average energy of an oscillator in one dimension = 2 × \(\frac{1}{2}\)KBT= KBT
Average energy of an oscillator in three dimension = 3KBT
For a mole of solid, the total energy is U = 3KBT × NA = 3RT (y R = NAKB)
Where NA = Avosadro number.
Question 7.
Indicate the graph showing the variation of specific heat of water with temperature. What does it signify?
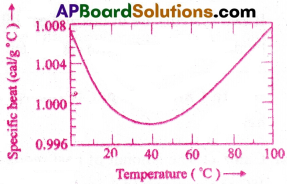
Answer:
It specifies the unit temperature interval as 14.5°C to 15.5°C for a precise definition of calorie.
Question 8.
Define state variables and equation of state. [Imp.Q]
Answer:
State variables :
Specific values of some microscopic variables like pressure, volume, temperature and mass which completely describe an equilibrium state of a thermodynamic system are known as state variables.
Equation of state :
The relation between the state variables is called the equation of state.
Ex: For an ideal gas, the equation of state is the ideal gas relation PV = nRT
Question 9.
Why a heat engine with 100% efficiency can never be realised in practice? [Imp.Q]
Answer:
The efficiency of heat engine is η = 1 – \(\frac{T_2}{T_1}\)
η is always less than one. The value of η can be one only if T2 = 0 . i.e if the sink is at absolute zero of temperature. Since the absolute zero of temperature can not be attained, therefore η can not be equal to one.
Question 10.
In summer, when the valve of a bicycle tube is opened, the escaping air appears cold, why? [Imp.Q]
Answer:
The pressure of air inside the tube is sufficiently greater than atmospheric pressure. When the valve of a bicycle tube is opened, the air expands under adiabatic process. It does some work against surroundings and so its internal energy decreases. This causes a fall in temperature. Hence escaping air from a bicycle tube appears cold.
Question 11.
Why does the brake drum of an automobile get heated up while moving down at constant speed? [Imp.Q]
Answer:
Since the speed is constant there is no change of kinetic energy. The loss in gravitational potential energy is partially the gain in the heat energy of the brake drum of an automobile while moving down at constant speed.
![]()
Question 12.
Can a room be cooled by leaving the door of an electric refrigerator open? [Imp.Q]
Answer:
No. The room can not be cooled by opening the door of an electric refrigerator.
A refrigerator extracts heat from the freezing chamber(cold reservoir), some work is done on it by electric motor and rejects heat into the surrounding(hot reservoir) air. If the door of a running refrigerator is left open, more heat is rejected to the surroundings. Hence the room will get slightly heated.
Question 13.
Which of the two will increase the pressure more, an adiabatic or an isothermal process, in reducing the volume to 50%?
Answer:
The pressure is more during an adiabatic process.
Isothermal compression :
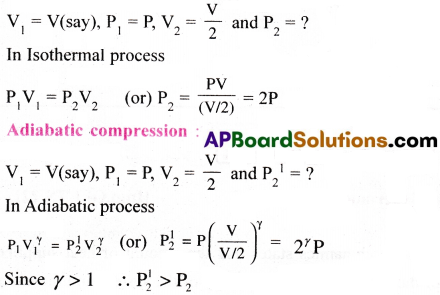
Question 14.
A thermos flask containing a liquid is shaken vigorously, what happens to its temperature? [Imp.Q]
Answer:
Heat is not added to the liquid externally, but work is done on the liquid. Since it is vigorously shaken the internal energy of the liquid increases, so the temperature of the system raises.
Question 15.
A sound wave is sent into a gas pipe. Does its internal energy change?
Answer:
Yes. The propagation of sound wave through a pipe is a quick process. It is an adiabatic change. So temperature increases. Therefore internal energy also increases.
Question 16.
How much will be the internal energy change in
i) isothermal process
ii) adiabatic process
Answer:
i) There is no change in the internal energy of an ideal gas in an isothermal process.
i.e dU = 0
ii) For adiabatic process dQ = 0
Hence dU = -dW
Work done by the gas results in decrease in its internal energy.
If work is done on the gas then its internal energy increases.
Question 17.
The coolant in si chemical or a nuclear plant should have high specific heat. Why? [Imp.Q]
Answer:
By allowing water to flow in pipes around the heated parts of a machine heat energy from such parts is removed. Water in pipes extracts more heat without much rise in its temperature because of its large specific heat.
![]()
Question 18.
Explain the following processes
i) isochoric process
ii) isobaric process
Answer:
Isochoric process :
A process that takes place at constant volume of the system is called Isochoric process.
Ex: Heat is added to the gas enclosed in a cylinder having rigid walls and a fixed piston.
Isobanc process :
A process that takes place at constant pressure of the system is called Isobaric process.
Ex: Heating of water at atmospheric pressure.
Short Answer Questions
Question 1.
State and explain first law of thermodynamics.
Answer:
First law of Thermodynamics:
Statement :
The first law of thermodynamics states that the amount of heat supplied to a system is equal to the sum of the change in internal energy of the system and the amount of external work done by the system.
If a small quantity of heat (dQ) is supplied to a gas, a part of it is used to increase internal energy(dU) and remaining heat is used in doing external work (dW).
Then dQ = dU + dW
Sign convention:
If the work is done by the system, dW is positive
If the work is done on the system, dW is negative
When heat is supplied to the system, dQ is positive
When the heat is taken out from the system, dQ is negative
The essential content of the first law of thermodynamics is that it defines one of the thermodynamic quantities ‘the internal energy’ or ‘internal energy function’.
This law is a consequence of conservation of energy.
Limitations:
- It does not tell about the direction of heat flow.
- It does not tell us about the efficiency with which heat can be converted into work.
Question 2.
Define two principle-specific heats of a gas. Which is greater and why? [TS 15]
Answer:
Specific heat at constant pressure (Cp) :
At constant pressure, the quantity of heat necessary to increase the temperature of unit mass of a gas through one degree, is called specific heat of the gas at constant pressure.
Specific heat at constant volume (Cv) :
At constant volume, the quantity of heat necessary to increase the temperature of unit mass of a gas through one degree, is called specific heat of gas at constant volume.
When heat is supplied to a system (gas) at constant volume, the heat is completely utilised to increase the internal energy of the system (gas).
But at constant pressure, supplied heat will he utilised in two ways :
- To increase the internal energy of the system.
- Todo work against external pressure (PdV).
Hence, more heat is to be supplied to the system at constant pressure than constant volume for the same rise of temperature. Hence Cp > Cv.
Question 3.
Derive a relation between the two specific heat capacities of gas on the basis of first law of thermodynamics, (or) Derive Cp – Cv = R. [TS 15]
Answer:
Consider one mole of Ideal Gas contained in a cylinder provided with a frictionless Piston.
Let Abe the area of cross-section of the Piston and P, V, T be the pressure, volume and temperature of the gas.
When Gas is heated, it expands and Piston moves up a distance dx.
Case – I :
Let the Gas be heated at constant volume, then Heat supplied is dQ1 = CvdT
From first law of thermodynamics, dQ = dU + dW
But, in this case dW = 0 ∴ dQ = dU = CvdT ………… (1)
Case – II :
Let the Gas be heated at constant pressure. then Heat supplied is dQ = CvdT ………… (2)
But, workdone by the gas is dW = F.S = (PA)dx = PdV, [Since, Adx = dV]
∴ dW = PdV ………….. (3)
From first law of thermo dynamics, dQ = dU + dW ………….. (4)
Substitute equations (1), (2), (3) in equation (4) we have CpdT = CvdT + PdV ……….. (5)
From Ideal gas equation PV = RT
At constant pressure, PdV= RdT
Hence, from (5), we get CpdT = CvdT + RdT ⇒ Cp = Cv + R
∴ Cp – Cv = R
![]()
Question 4.
Obtain an expression for the work done by an ideal gas during isothermal change. [Imp.Q]
Answer:
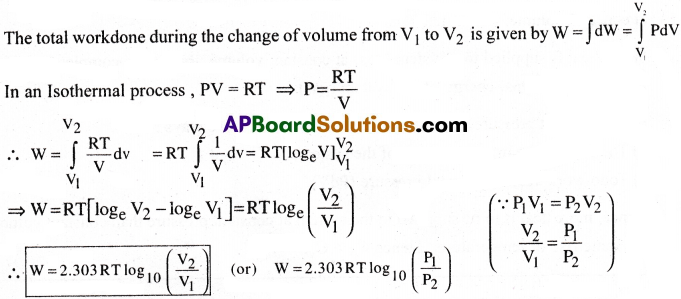
Isothermal Process :
The process in which pressure and volume changes occur at constant temperature is called Isothermal process.
Workdone :
Let a certain mass of gas expands from volume V1 to V2 and pressure changes from P1 to P2 isothermally at constant temperature T.
Work done by the gas during a small change in volume is dW = PdV.
Question 5.
Obtain an expression for the work done by an ideal gas during adiabatic change and explain. [Imp.Q]
Answer:
Adiabatic Process :
The process in which changes in pressure and volume of a gas takes place at constant Heat energy is called Adiabatic process.
Let a certain mass of gas expand adiabatically from volume V1 to V2.
Let pressure changes from P1 to P2 and temperature T1 to T2.
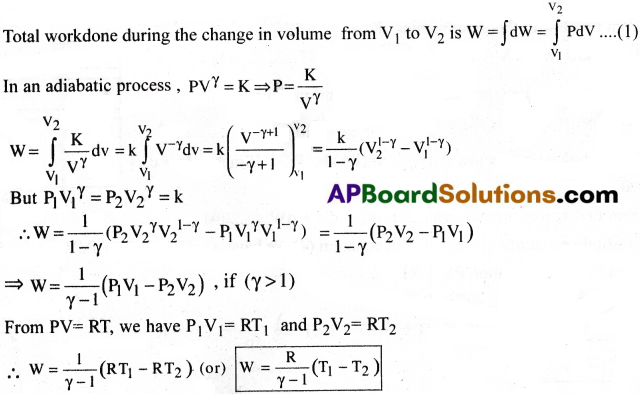
Question 6.
Compare isothermal and an adiabatic process.
Answer:
| Isothermal Process | Adiabatic Process |
| 1) In Isothermal process, temperature remains constant and heat exchanges between system and surroundings. | 1) In Adiabatic process, total heat remains constant and temperature changes between system and surroundings. |
| 2) This process should be performed in a good conducting vessel. | 2) This process should be performed in a bad conducting vessel. |
| 3) It is a slow process. | 3) It is a quick process |
| 4) Gas law PV = constant,holds true. | 4) Gas law PVγ = constant, holds true. |
| 5) Specific heat is infinity. | 5) Specific heat is zero. |
| 6) External work done is zero (dW = 0). | 6) There is some external work done (dW ≠ O) |
| 7) First law of Thermodynamics becomes dQ = dU |
7) First law of Thermodynamics becomes dU + dW = 0(dQ = 0). |
| 8) Ex: Boiling of water in open air. | 8) Ex: Compressing an ideal gas quickly. |
![]()
Question 7.
Explain the following processes [Imp.Q]
i) Cyclic process with example.
ii) Non cyclic process with example.
Answer:
Cyclic process:
A process in which the system after passing through various stages (pressure, volume, and temperature changes) returns to its initial state is defined as a cyclic process.
The internal energy U of the system depends only on the state of the system and not on the path followed. As we reach finally the initial state in a cyclic process, there will be no change in the internal energy, i.e dU = 0.
According to first law of thermodynamics, for a cyclic process, dQ = dW.
That is in a cyclic process, the total heat absorbed by the system equals the work done by the system.
Ex: 1) The Carnot cycle is the best example of the cyclic process
2) A system in heat engine is made to undergo a cyclic process.
Non cyclic process:
Non cyclic process is that process in which the system does not return to its initial stage.
Ex: When ice is placed in a given space warmed than 0°C, heat flows into the ice and the space is cooled (or) refrigerated. The latent heat of fusion of ice is supplied from the surroundings and the ice changes its state. This refrigeration effect has been accomplished by non-cyclic process.
Question 8.
Write a short note on Quasi-static process.
Answer:
The thermodynamic variables like pressure(P), volume(V), temperature(T) and mass(m) characterise a thermodynamic system.If these variables do not change with time then the system is in thermodynamic equilibrium. If the pressure of the gas equals the external pressure and the temperature of the gas is also the same as that of the surrounding then gas is in thermodynamic equilibrium with its surroundings.
If the piston is pushed downwards, the gas inside the vessel will undergo sudden compression. During the compression due to rapid change in pressure and temperature, the gas passes through several non-equilibrium states and finally attains equilibrium state with the surrounding. Imagine an idealised process in which at every stage the system is in all equilibrium state. Such a process should take place in a very slow manner and at every stage the process appears to be almost nearly static and such an idealised process is called quasi-static process.
At every stage the system will be in thermal and mechanical equilibrium with its surroundings. Temperature difference and pressure differences will be infinitesimally small.
Definition:
A quasi-static process can be defined as an infinitesimally slow process in which at each and every intermediate stage the system remains in thermal and mechanical ( thermodynamic) equilibrium with the surroundings through out the entire process.
In practice we can treat any process taking place sufficiently slowly, not involving accelerated motions and large temperature gradients can be considered to be a quasi-static process.
Question 9.
Explain qualitatively the working of a heat engine. [Imp.Q]
Answer:
Heat Engine :
A device used to convert heat energy into work is called a heat engine. A heat engine consists of three parts.
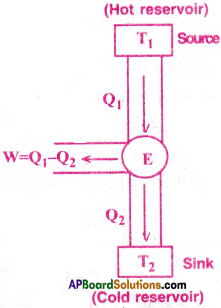
(i) Source :
Source is the body at a higher temperature(T1).
Heat (Q1) is extracted from this body and hence it is called “source”.
(ii) Working substance :
Ideal gas acts as a working substancein a heat engine.
Ex: In a steam engine, the working substance is steam.
(iii) Sink :
Sink is the body at a lower temperature(T2)
Heat (Q2) is rejected by the working substance into the sink.
∴ Workdone by the steam is W = Q1 – Q2
Efficiency of the Heat Engine:
The efficiency (r)) of a heat engine defined as, the ratio of the work (W) done by the engine to the amount of Heat(Q1) absorbed by the engine
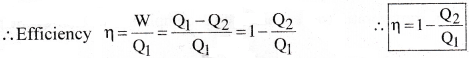
Long Answer Questions
Question 1.
Explain reversible and irreversible processes. Describe the working of Carnot engine. Obtain an expression for the efficiency. |TS 15, 17, 19, 19; AP 16, 17, 18, 19, 20, 22]
Answer:
Reversible process :
A process that can be retraced back in the opposite direction is called a reversible process.
Ex: Fusion of ice and vaporisation of water.
Irreversible process :
A process that can not be retraced back in the opposite direction is called an irreversible process.
Ex: Work done against friction.
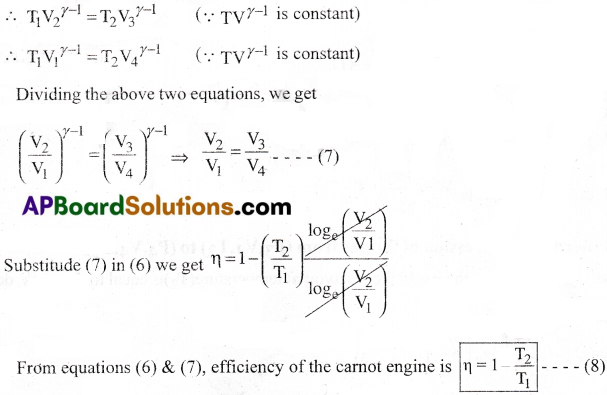
Carnot engine :
Areversible heat engine operating between two temperatures is called a Carnot engine.
Working of Carnot engine :
The carnot engine undergoes a cycle of processes called camot cycle. It consists of two isothermal processes connected by two adiabatic processes. Ideal gas acts as the working substance in the camot engine.
The steps of Carnot cycle:
a) Isothermal expansion of the gas taking its state from (P1,V1, T1) to (P2, V2, T2).
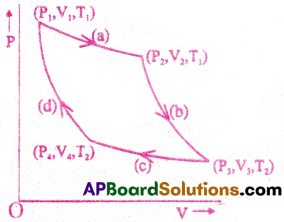
Heat(Q1) absorbed by the gas from the reservoir at temperature (T1) is equal to the work done (W1) by the gas.
W1 = Q1 = nRT1loge(\(\frac{V_2}{V_1}\)) ——– (1)
b) Adiabatic expansion of the gas from (P2,V2, T1) to (P3, V3, T2).
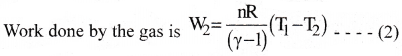
c) Isothermal compression of the gas from (P3, V3, T2) to (P4, V4, T2).
Heat (Q2) released by the gas to the reservoir at temperature(T2)is equal to the work done on the gas .
W3 = Q2 = nRT2loge(\(\frac{V_2}{V_1}\)) ——– (3)
d) Adiabatic compression of the gas from (P4, V4, T2) to (P1, V1, T1).
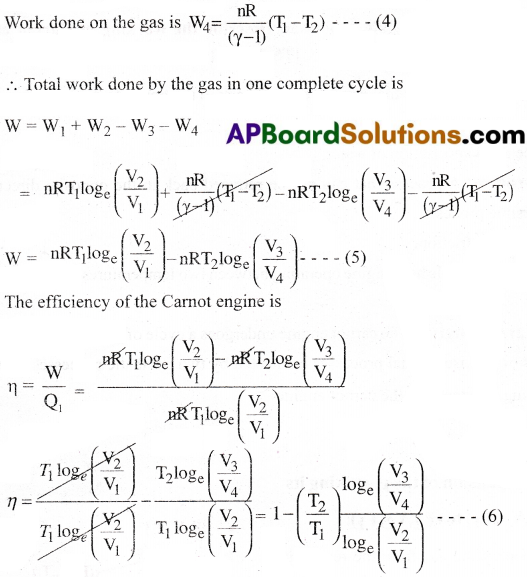
(b) & (d) sire adiabatic processes.
![]()
Question 2.
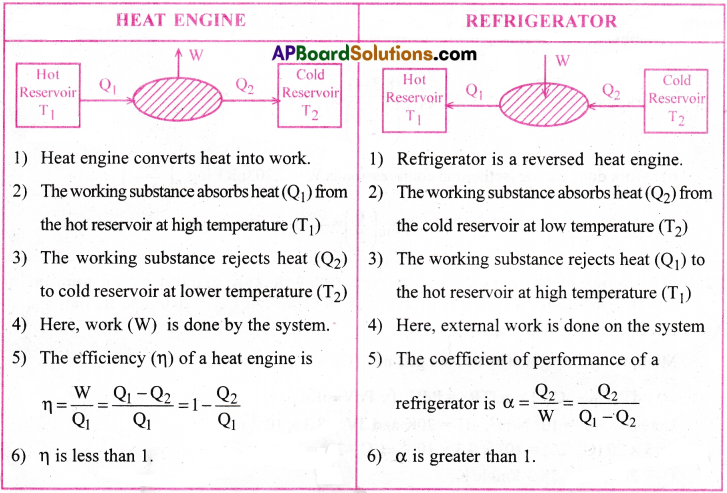
State second Saw of thermodynamics. How is heat engine different from a refrigerator. [AP 19; TS 18, 20]
Answer:
Second law of thermodynamics:
Second law of thermodynamics gives the direction of flow of heat.
It consists of two statements.
I) Kelvin – Plank Statement :
It is impossible to construct a heat engine that absorbs heat from a hot reservoir which converts completely that heat into work.
(or) It is impossible to construct an ideal heat engine with 100% thermal efficiency,
II) Clausius Statement :
It is impossible to transfer heat from a colder object to a hotter object (or) It is impossible to construct an ideal refrigerator.
Exercise Problems
Question 1.
If a monoatomic ideal gas of volume 1 litre at N.T.P is compressed (i) adiabatically to half of its volume, find the work done on the gas. Also find (ii) the work done if the compression is isothermal.(γ = 5/3)
Answer:
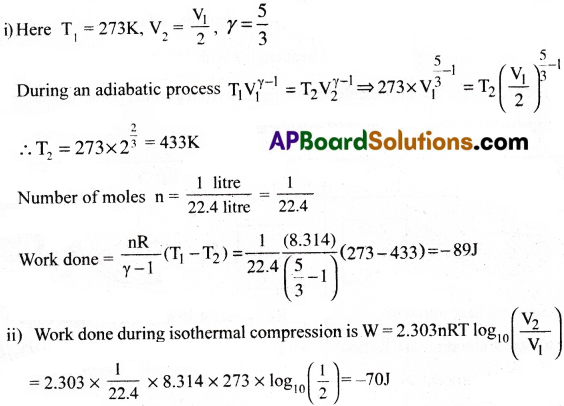
Question 2.
Five moles of hydrogen when heated through 20K expand by an amount of 8.3 × 10-3 m³ under a constant pressure of 105 N/m². If CV = 20J/mole K, find CP.
Answer:
Cp – Cv = R
Multiplying throughout by ndT we get, ndT(CP – CV) = ndTR
⇒ ndT(CP – CV) = ndTR = PdV (∵ PdV = nRdT)
Here n = 5, P = 105 N/m², dT = 20K and dV = 8.3 × 10-3 m³
⇒ 5 × 20 (CP – 20) = 105 × 8.3 × 10-3 ⇒ CP – 20 = 8.3
CP = 20 + 8.3 = 28.3 J/mole K
Question 3.
A refrigerator is to maintain eatables kept inside at 9°C. If room temperature is 36°C, calculate the coefficient of performance.
Answer:
Here T, = 273 + 36 = 309 K, T2 = 273 + 9 = 282 K 282 282
Question 4.
What amount of heat must be supplied to 2.0 × 10-2 kg of nitrogen (at room temperature) to raise its temperature by 45°C at constant pressure?
(Molecular mass of N2 = 28, R = 8.3 ,1/ mol. K) [AP 20]
Answer:
Amount of heat supplied at constant pressur dQP = nCPdT

Question 5.
A cylinder with a movable piston contains 3 moles of hydrogen at standard temperature and pressure. The walls of the cylinder are made of a heat insulator and the piston is insulated by having a pile of sand on it. By what factor does the pressure of the gas increase if the gas is compressed to half its original volume?
Answer:

The pressure of the gas increases by a factor 2.64 when the gas is compressed to half its original volume.
Question 6.
In changing the state of a gas adiabatically from an equilibrium state A to another equilibrium state B an amount of work equal to 22.3 J is done on the system. If the gas is taken from state A to B via a process in which the net heat absorbed by the system is 9.35 cal how much is the net work done by the system in the latter case?
(Take I cal = 4.19 J)
Answer:
The system goes from state A to state B adiabatically. ∴ dQ = 0
According to first law of thermodynamics dQ = dU + dW ⇒ 0 = dU + dW ⇒ dU= -dW
The work done on the system dW= -22.3 J
∴ dU = -(-22.3) = 22.3 J
In the second case, ∆Q = 9.35 cal = 9.35 × 4.19 = 39.18 J
∴ dQ = dU + dW ⇒ dW = dQ – dU= 39.18 – 22.3 = 16.88 J
Question 7.
An electric heater supplies heat to a system at a rate of 100W. If system performs work at a rate of 75 joules per second. At what rate is (he internal energy increasing?
Answer:
Rate of heat supplies to the system, \(\frac{dQ}{t}\) = 100w = 100 Js-1
Rate of work done by the system, \(\frac{dQ}{t}\) = 75 JS-1
According to first law of thermodynamics,
![]()
The rate of increase in internal energy = 25 JS-1
Question 8.
Find the external work done by the system in kcal, when 20 kcal of heat, is supplied to the system the increase in its internal energy is 8400 J. (J = 4200 J/kcal).
Answer:
From, the first law of thermodynamics, dQ = dU + dW
Given dU = 8400J = \(\frac{8400}{4200}\) = 2kcl, dQ = 20kcl
The external work done dW = dQ – dU= 20 – 2 = 18 kcal
![]()
Question 9.
Find the efficiency of a heat engine if the temperature of the source is 100°C and sink is 27°C.
Answer:
Formula: η = 1 – \(\frac{T_2}{T_1}\)
Given T1 = 373K, T2 = 300K
∴ η = 1 – \(\frac{300}{373}\) = 1 – 0.8043 = 0.1957 (or) η = 19.57 %
Multiple Choice Questions
Question 1.
There are two lead spheres at the same temperature, the ratio of radii being 1 : 2. The ratio of heat capacities are
1) 1 : 2
2) 1 : 4
3) 1 : 6
4) 1 : 8
Answer:
4) 1 : 8
Question 2.
5Kg of ice at -10°C is added to 5 Kg of water at 10°C. The temperature of the resulting mixture will be
1) -10°C
2) 0°C
3) 3.3°C
4) 10°C
Answer:
2) 0°C
Question 3.
A diatomic gas molecule has translational, rotational and no vibrational degrees of freedom. The ratio of specific heats CP/CV
1) 1.67
2) 1.4
3) 1.29
4) 1.33
Answer:
2) 1.4
Question 4.
An amount of water of mass 20gms at 0°C is mixed with 40gms of water at 10°C. Final temperature of mixture is
1) 20°C
2) 6.66°C
3) 5°C
4) 0°C
Answer:
2) 6.66°C
Question 5.
The pressure and density of a diatomic gas (γ = \(\frac{7}{5}\)) change adiahatically from (P,d) to (P¹d¹).If \(\frac{d^1}{d}\) = 32, then \(\frac{P^1}{P}\) is
1) \(\frac{1}{128}\)
2) 32
3) 128
4) 256
Answer:
3) 128
![]()
Question 6.
A gas at 250K is suddenly compressed to 1/8 of its original volume. The rise in temperature is
1) 321 K
2) 421 K
3) 621 K
4) 871 K
Answer:
3) 621 K
Question 7.
Time taken by a 836W heater to heat one litre of water from 10°C to 40°C is
1) 50 s
2) 100 s
3) 150 s
4) 200 s
Answer:
3) 150 s
Question 8.
For an isothermal expansion of a perfect gas the value of \(\frac{\Delta \mathrm{P}}{\mathrm{P}}\) is equal to
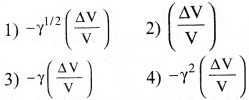
Answer:
2
Question 9.
During an adiabatic process, the pressure of a gas is found to be proportional to the cube of its absolute temperature. The ratio of CP/CV for the gas is
1) 4/3
2) 2
3) 5/3
4) 3/2
Answer:
4) 3/2
Question 10.
One mole of ideal monoatomic gas (γ = 5/3) is mixed with one mole of diatomic gas (γ = 7/5) . What is γ for the mixture? [γ denotes the ratio of specific heat at constant pressure, to that at constant volume]
1) 3/2
2) 23/15
3) 35/23
4) 4/3
Answer:
1) 3/2
Question 11.
First law of thermodynamics is consequence of conservation of
1) work
2) energy
3) heat
4) all of these
Answer:
2) energy
Question 12.
The internal energy change in a system that has absorbed 2 kcal of heat and done 500 J of work is
1) 6400 J
2) 5400J
3) 7900J
4) 8900J
Answer:
3) 7900J
![]()
Question 13.
110 joule of heat is added to a gaseous system whose internal energy is 40 J, then the amount of external work done is
1) 150 J
2) 70 J
3) 110 J
4) 40 J
Answer:
2) 70 J
Question 14.
A monatomic gas at pressure P1 and volume V1 is compressed adiabatically to 1/8th of its original volume. What is the final pressure of the gas?
1) 64 P1
2) P1
3) 16P1
4) 32P1
Answer:
4) 32P1
Question 15.
If ∆U and ∆W represent the increase in internal energy and work done by the system respectively in a thermodynamical process, which of the following is true?
1) ∆U = – ∆W, in an adiabatic process
2) ∆U = ∆W, in an isothermal process
3) ∆U = ∆W, in an adiabatic process
4) ∆U = -∆W, in an isothermal process
Answer:
1) ∆U = – ∆W, in an adiabatic process
Question 16.
An ideal gas at 27°C is compressed adiabaticaliy to 8/27 of its original volume. The rise in temperature is (Take γ = 5/3)
1) 275 K
2) 375 K
3) 475 K
4) 175 K
Answer:
2) 375 K
Question 17.
An ideal gas, undergoing adiabatic change, has which of the following pressure-temperature relationship?
1) PγT1-γ = constant
2) P1-γTγ = constant
3) Pγ-1Tγ = constant
4) PγTγ-1 = constant.
Answer:
2) P1-γTγ = constant
Question 18.
In an adiabatic change, the pressure and temperature of a monatomic gas arc related as P ∝ TC, where C equals
1) 3/5
2) 5/3
3) 2/5
4) 5/2
Answer:
4) 5/2
Question 19.
Which of the following relations does not give the equation of an adiabatic process, where terms have their usual meaning?
1) p1-γTγ – constant
2) PVγ – = constant
3) TVγ-1 = constant
4) PγT1-γ =constant
Answer:
4) PγT1-γ =constant
![]()
Question 20.
A monatomic gas at a pressure P, having a volume V expands isothermally to a volume 2V and then adiabaticaliy to a volume 16V. The final pressure of the gas is (Take γ = 5/3)
1) 64P
2) 32P
3) P/64
4) 16P
Answer:
3) P/64
Question 21.
One mole of an ideal monatomic gas undergoes a process described by the equation PV³ = constant. The heat capacity of the gas during this process is
1) 3/2 R
2) 5/2 R
3) 2R
4) R
Answer:
4) R
Question 22.
A mass of diatomic gas (γ = 1.4) at a pressure of 2 atmospheres is compressed adiabaticaliy so that its temperature rises from 27°C to 927°C. The pressure of the gas in the final state is
1) 8 atm
2) 28 atm
3) 68.7 atm
4) 256 atm
Answer:
4) 256 atm
Question 23.
A diatomic gas initially at 18°C is compressed adiabaticaliy to one eighth of its original volume. The temperature after compression will be
1) 395.4°C
2) 144°C
3) 18°C
4) 887.4°C
Answer:
1) 395.4°C
Question 24.
During an adiabatic process, the pressure of a gas is found to be proportional to the cube of its temperature. The ratio of Cp/Cv for the gas is
1) 5/3
2) 3/2
3) 4/3
4) 2
Answer:
2) 3/2
Question 25.
If cp and cv denote the specific heats per unit mass of an ideal gas of molecular weight M, then
where R is the molar gas constant.
1) Cp – Cv = R/M²
2) Cp – Cp = R
3) Cp – Cv = R/M
4) Cp – Cp = MR
Answer:
3) Cp – Cv = R/M
Question 26.
If the, ratio of specific heat of a gas at constant pressure to that at constant volume is y, the change in internal energy of a mass of gas, when the volume changes from V to 2V at constant pressure P, is
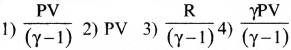
Answer:
1
Question 27.
One mole of an ideal gas requires 207 J heat to rise the temperature by 10 K when heated at constant pressure. If the same gas is heated at constant volume to raise the temperature by the same 10 K, the heat required is ((liven the gas constant R = 8.3 J/mole K)
1) 198.7 J
2) 29 J
3) 215.3 J
4) 124 J
Answer:
4) 124 J
Question 28.
In which of the following processes, heat is neither absorbed nor released by a system?
1) isochoric
2) isothermal
3) adiabatic
4) isobaric
Answer:
3) adiabatic
Question 29.
A thermodynamic system is taken from state A to B along ACB and is brought back to A along BDA as shown in the PV diagram.
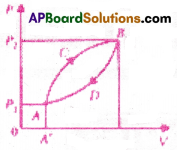
The net work done during the complete cycle is given by the area
1) P1ACBP2P1
2) ACBB’A’A
3) ACBDA
4) ADBB’A’A
Answer:
3) ACBDA
![]()
Question 30.
Which of the following processes is reversible?
1) Transfer of heat by conduction
2) Transfer of heat by radiation
3) Isothermal compression
4) Electrical heating of a nichrome wire
Answer:
3) Isothermal compression
Question 31.
An ideal gas heat engine operates in Carnot cycle between 227°C and 127°C. It absorbs 6 × 104 cal of heat at higher temperature. Amount of heat converted to work is
1) 4.8 × 104 cal
2) 6 × 104 cal
3) 2.4 × 104 cal
4) 1.2 × 104cal
Question 32.
An ideal Carnot engine, whose efficiency is 40%, receives heat at 500 K. If its efficiency is 50%, then the intake temperature for the same exhaust temperature is
1) 800 K
2) 900 K
3) 600 K
4) 700 K
Answer:
3) 600 K
Question 33.
A scientist says that the efficiency of his heat engine which work at source temperature 127°C and sink temperature 27°C is 26%, then
1) it is impossible
2) it is possible but less probable
3) it is quite probable
4) data are incomplete
Answer:
1) it is impossible
Question 34.
The efficiency of a Carnot engine operating with reservoir temperature of 100°c and -23°C will be

Answer:
2
Question 35.
When a heat Q is supplied to one mole of mono-atomic gas (γ = 5/3) then molar heat capacity of the gas at constant volume is
![]()
Answer:
1
Question 36.
The temperature of 5 moles of a gas at a constant volume is changed from 100°C to 120°C. The change in internal energy is 80J. The heat capacity of the gas at constant volume will be ( in J/K)
1) 8
2) 4
3) 0.8
4) 0.4
Answer:
2) 4
![]()
Question 37.
When a mono-atomic gas expands at constant pressure, the percentage of heat supplied that increase the internal energy of the gas and that which is involved in expansion is
1) 75%, 25%
2) 25%, 75%
3) 60%, 40%
4) 40%, 60%
Answer:
3) 60%, 40%
Question 38.
PV diagram of an ideal gas is shown in figure. Work done by the gas in the process ABCD is
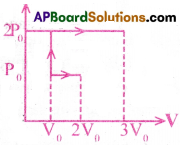
1) 4P0V0
2) 2P0V0
3) 3P0V0
4) P0V0
Answer:
3) 3P0V0