Access to a variety of AP Inter 1st Year Chemistry Model Papers and AP Inter 1st Year Chemistry Question Paper March 2020 helps students overcome exam anxiety by fostering familiarity. question patterns.
AP Inter 1st Year Chemistry Question Paper March 2020
Note : Read the following instructions carefully.
- Answer all questions of Section ‘A’. Answer any six questions in Section ‘B’ and any two questions in Section ‘C’.
- In Section ‘A’, questions from Sr. Nos. 1 to 10 are of “Very Short Answer Type”. Each question carries two marks. Every answer may be limited to 2 or 3 sentences. Answer all these questions at one place in the same order.
- In Section ‘B’, questions from Sr. Nos. 11 to 18 are of “Short Answer Type”. Each question carries four marks. Every answer may be limited to 75 words.
- In Section ‘C’, questions from Sr. Nos. 19 to 21 are of “Long Answer Type”. Each question carries eight marks. Every answer may be limited to 300 words.
- Draw labelled diagrams wherever necessary for questions in Sections ‘3’ and ‘C’.
Section – A
Note : Answer all questions.
Question 1.
What is Bio-chemical oxygen demand [BOD]?
Answer:
The amount of oxygen used by the suitable micro organisms present in water during five days at 20°C is called as Bio Chemical Oxygen Demand (BOD).
Question 2.
Write names of the any four gases which causes green-house effect.
Answer:
CO2, CFC, CH4, O3, N2O, water vapour etc. – are green house gases.
Question 3.
Why does the solubility of alkaline earth metal hydroxides in water increase down the group.
Answer:
Among alkaline earth metal hydroxides, the anion being common the cationic radius will influence the lattice enthalpy. Since, lattice enthalpy decreases much more than the hydration enthalpy with increasing ionic size, the solubility increases as we go down the group.
![]()
Question 4.
Describe the important uses of sodium carbonate.
Answer:
Uses:
Na2CO3 is used in the manufactuing of glass.
Na2CO3 is used in the manufactuing of borax, caustic soda.
Na2CO3 is used in paper, paints and tetfile industries.
Na2CO3 is used: in softening of water.
Na2CO3 is used in laundries.
Na2CO3 is an important laboratory reagent both in qualitative and quantiative analysis.
Question 5.
On a ship sailing in pacific ocean where temperature is 23.4°C. a ballon is filled with 2 litre air. What will be the volume of the ballon when the ship reaches Indian Ocean where temperature is 26.1°C?
Answer:
\(\frac{V_1}{T_1}=\frac{V_2}{T_2}\)
V<sub>2</sub>\(\frac{V_1 T_2}{T_1}=\frac{2 \times 299.1}{296.4}\) = 2.018L
Question 6.
Calculate the normality of oxalic acid solution containing 6.3 gm of H2C2O4 2H2O in 500 ml solution.
Answer:
Normality (N) = \(\frac{W}{G \sum W} \times \frac{1000}{V(m s)}\)
= \(\frac{6.3}{63} \times \frac{1000}{500}\) = 0.2N
Question 7.
Give two chemical equilibrium reactions for which kp > kc.
Answer:
N2 O4(g) ⇌ 2NO2(g) (Kp > KC)
PCl5(g) ⇌ PCl3(g) + Cl2(g) (Kp > KC)
Question 8.
What is allotropy? Give the crystalline allotropes of carbon.
Answer:
- The phenomenon of existence of an element in different physical forms having similar (or) same chemical properties is called allotropy.
- Crystalline allotropes of carbon are
a) Diamond
b) Graphite
c) Fullerences.
![]()
Question 9.
What is ‘synthesis’ gas ? How it is. prepared?
Answer:
A mixture of Co and H2 is called syh=theSfs’gas.
It is prepared by passing super heated steam over hot coke.
![]()
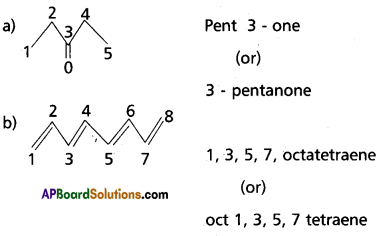
Question 10.
Write the IUPAC name of

Answer:
Section – B
Question 11.
Define Dipole moment. Write its applications.
Answer:
Dipole moment: The product of magnitude of the charge and the distance between the two poles (bond length) is called dipole moment.
Dipole moment μ = q x d
q = charge
d = bond length
Units : Debye (D), 1 Debye = 3.34 × 10-30 coulombs metres.
Applications :
Dipole moment is used to calculate the percentage of Ionic character in a molecule.
It’is used to know the shape of the molecule.
Symmetry (symmetrical (or) non symmetrical) of the molecule can be known by dipole moment.
![]()
Question 12.
Explain the hybridisation involved in PCl5 molecule.
Answer:
1) In PCl5 the electron configuration of phosphoru is
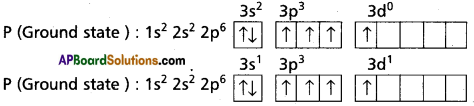
2) Phosphorus undergoes sp3d – hybridisation by intermixing of one s-orbital [3s], three p – orbitals [3px, 3py, 3pz] and one d – orbital.
These five hybrid orbitals overlap. The pz orbitals of chlorine atoms forming five σsp5d-s bonds. Out of these five p – cl bonds three are coplanar and the remaining two are in the axial position. There by PCl5 acquires the trigonal bipyramidal shape. The molecule contains two bond angles 90° and 120°. .
Question 13.
Deduce:
a) Graham’s law and
b) Daltons law from kinetic gas equation.
Answer:
a) Graham’s law : At constant temperature and pressure the rate of diffusion of a gas in inversely proportional to the square root of its density, r ∝ \(\frac{1}{\sqrt{d}}\)
Deduction : kinetic gas equation is
PV = \(\frac{1}{3}\) mnu2 = \(\frac{1}{3}\) Mu2
U2 = \(\frac{3 P V}{M}=\frac{3 P}{d}\)
∴ u = \(\sqrt{\frac{3 P}{d}}\)
At constant pressure u = k \(\frac{1}{\sqrt{d}}\)
K = constant Or u ∝ \(\frac{1}{\sqrt{d}}\)
∵ The rate diffusion of gases depends upon the velocity of the gas molecules.
So r ∝ \(\frac{1}{\sqrt{d}}\)
This is graham’s law.
b) Dalton’s law of partial pressures:
The total pressure exerted by a mixture of chemically non-reacting gases at given temperature and volume, is equal to the sum of the partial pressures of the component gases.
Deduction :
Consider a gas present in vessel of volume = V
no. of molecules = n1
mass of each molecule = m1
RMS velocity = u1
According to kinetic gas equation the pressure of the gas.
p1 = \(\frac{1}{3} \frac{m_1 n_1 u_1^2}{V}\)
When this gas is replaced by another gas in the same vessel,
p2 = \(\frac{1}{3} \frac{m_2 n_2 u_2^2}{v}\)
When these two gases are mixed in the same vessel, the total pressure of the mixture is
p = \(\frac{1}{3} \frac{m_1 n_1 u_1^2}{V}\) + \(\frac{1}{3} \frac{m_2 n_2 u_2^2}{v}\) = p1 + p2
∴ p = p1 + p2
This is Dalton’s law of partial presssures.
Question 14.
Balance the following redox reaction by ion – electron method in basic medium.
Answer:
Given
MnO4– Br– → MnO2 + BrO3
| Reduction half cell | Oxidation half cell |
| MnO4– → MnO2 | Br– → BrO3– |
| MnO4– → MnO2 + 2H2O | Br– + 3H2O → >BrO3– |
| MnO4– + 4H2O → MnO2 + 2H2O + 4OH– | Br– + 3H2O + 6OH– → BrO3– + 6H2O |
| MnO4– + 2H2O → MnO2 + 4OH– | Br– + 6OH– → BrO3– + 3H2O |
| MnO4– + 2H2O + 3e– → MnO2 + 4OH– | Br– + 6OH– → BrO3– + 3H2O + 6e– |
Combining Eq (1) and (2)
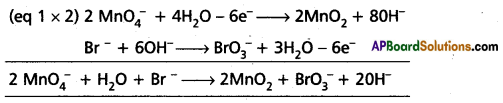
Question 15.
State and explain the Hess’s law of constant heat summation. Answer: Hess’s law states that the total amount of heat evolved or absorbed in a chemical reaction is always same whether the reaction is carried out in one step (or) in several steps.
Illustration : This means that the heat of reaction depends only on the initial and final stages and not on the intermediate stages through which the reaction is carried out. Let us consider a reaction be ∆H.
A → B : ∆H1
B → C : ∆H2
C → D : ∆H3
The net heat of reaction is ∆H1 + ∆H2 + ∆H3.
According to Hess law AH = ∆H1 + ∆H2 + ∆H3.
Ex: Consider the formation of CO2. It can be prepared in two ways.
1) Direct method : By heating carbon in excess of O2.
C(S) + O2(g) → CO2(g); ∆H = – 393.5 kJ
2) Indirect method : Carbon can be converted into CO2 in the following two steps.
C(S) + \(\frac{1}{2}\) O2(g) → CO2(g); ∆H1 = – 110.5 kJ
C(g) + \(\frac{1}{2}\) O2(g) → CO2(g); ∆H2 – – 283.02 kJ
Total ∆H = – 393.52 kJ (∆H1 + ∆H2)
The two AH values are same.
![]()
Question 16.
Write the conjugate acid and conjugate base of each of the following :
(a) OH–
(b) H2O
(c) HCO3–
(d) H2O2
Answer:
| Given species | Conjugate acid | Conjugate base |
| (a)OH– | H2O | O-2 |
| (b) H2O | H3O+ | OH– |
| (c) HCO3– | H2CO3 | CO3-2 |
| (d) H2O2 | H3O2+ | HO2– |
Question 17.
Discuss the position of hydrogen in the periodic table on the basis of its electronic conftgUratfon.
Answer:
Hydrogen is the simplest element with one electron and proton, its electronic configuration Us is. The configuration is responsible for its dual nature. It behaves both UkealKali metals and halogens. So, it can be placed aloncj With alkali imetals (IA) or along with halogens (VIIA).
Points in support of piacina it in IA group, a) Like alkali metals hydrogen also has a single electron in the outer shell 1s-1.
b) Its ability to form hydrated unipositive ion, H+ (aq).
c) It is quite reasonable to start the periodic table with an element having the least atomic number (Z = 1).
Points in support of placing it in VIIA group.
a) Hydrogen is a gas like fluorine or chlorine.
b) It can form diatomic molecule like halogens.
c) It has a tendency of gaining an electron and attains a stable electronic configuration of He forming H” ion like halogens.
At the same time it should be noted that hydrogen has not such a great tendency to lose electron like alkali metals and gain an electron like halogens. In view of this it is difficult to assign any definite position to hydrogen. Sometimes it is placed in IA group and sometime with VIIA group.
Question 18.
Explain borax bead test with suitable example.
Answer:
Borax bend test: This test is useful in the identification of basic radicals in qualitative analysis. On heating borax swells into a white, opaque mass of anhydrous sodium tetra borate. When it is fused, borax glass is obtained. Borax glass is sodium meta borate and B2O3. The boric anhydride, B2O3, combined with metal oxides to form metal metaborates as coloured beads. The reactions are as follows :.
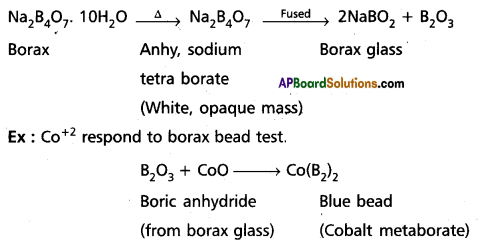
Question 19.
What are the postulates of Bohr’s model of hydrogen atom ?Discuss the importance of this model to explain various series of line spectra in hydrogen atom.
Answer:
Niels Bohr quantitatively gave the general features of hydrogen atom structure and it’s spectrum. His theory is used to evaluate several points in the atomic structure and spectra.
The postulates of Bohr atomic model for hydrogen as follows:
Postulates :
The electron in the hydrogen atom can revolve around the nucleus in a circular path of fixed radius and energy. These paths are called orbits (or) stationary states. These circular orbits are concentric (having same center) around the nucleus. The energy of an electron in the orbit does not change with time.
When an electron moves from lower stationary state to higher stationary state absorption of energy takes place.
When an electron moves from higher stationary state to lower stationary state emission of energy takes place.
When an electronic transition takes place between two stationary states that differ in energy AE is given by ∆E = E2 – E1 = hν
∴ The frequency of radiation absorbed (or) emitted ν = \(\frac{E_2-E_1}{h}\) E1 and E2 are energies of lower, higher energy states respectively.
The angular momentum of an electron is given by mvr = \(\frac{\mathrm{nh}}{2 \pi}\)
An electron revolve only in the orbits for which it’s angular momentum is integral multiple of \(\frac{h}{2 \pi}\)
Line spectra of Hydrogen – Bohr’s Theory :
Incase of hydrogen atom line spectrum is observed and this can be explained by using Bohr’s theory.
According to Bohr’s postulate when an electronic transition takes place between two stationary states that differ in energy is given by
∆E = Ef – Ei
Ef = final orbit energy
Ei = initial orbit energy
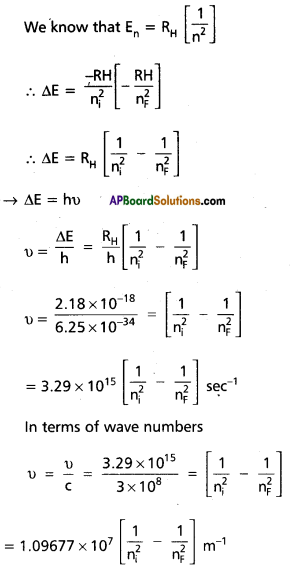
- Incase of absorption specturm nf > ni → energy is abosorbed (+Ve)
- Incase of emission specturm nf > ni → energy is abosorbed (+Ve)
- Each spectral line in absorption (or) emission spectrum associated to the particular transition in yogrogen atom
- Incase of large no.of hydrogen atoms large np.of transitions possible they results in large no.of spectral lines. The series of lines observed Transitions of the electron in the hydrogen atom
| ‘n’ value | Series | Region |
| 1. | Lyman series | UV region |
| 2. | Baimer series | visible |
| 3. | Paschen series | Near I.R. |
| 4. | Brackette series | I.R. |
| 5. | Pfund series | Far I.R. |
![]()
Question 20.
Write an essay on s, p, d and f block elements.
Answer:
According to the electronic configuration of elements, have been classified into four blocks. The basis for this classification is the entry of the differentiating electron into the subshell. They are classified into s, p, d and f blocks.
‘S’ block elements : If the differentiating electron enters into ‘s’ orbital, the elements belongs to ‘s’ block.
In every groupthere are tvvo ‘s’ block elements. As an.’s’ orbital can. have, a,maximum of two electrons, ‘s’ block has two groups IA an IIA. ‘P’ block elements : If the differentiating electron enters into ‘p’
‘p’ block contains six elements in each period. They are IIIA to VII A and zero group elements. The electronic configuration of ‘p’ block elements varies from ns2np1 to ns2 np6.

‘d’ block elements :
It the differentiating electron enters into (n -1) d – orbitals the elements belongs to ‘d’ block. These elements are in between ‘s’ and ‘p’ blocks. These elements are also known as transition elements. In these elements n and (n – 1) shells are incompletely filled. The general electronic configuration of ‘d‘ block elements is (n -1) d1-10 ns1-2. This block consists of IIIB to VIIB, VIII, IB and MB groups.
f block elements :
If the differentiating electron enters into T orbitals of antipenultimate shell (n – 2) of atoms of the elements belongs to ‘f block. They are in sixth and seventh periods in the form of two series with 14 elements each. They are known as lanthanides and actinides and are arranged at the bottom of the periodic table. The general electronic configuration is (n – 2) f1-14 (n – 1) d0-1sn2.
In these shells the last three shells (ultimate, penultimate and antf penultimate) are incompletely filled. Lanthanides belongs to 4f series. It contains Ce to Lu. Actinides belong to 5f series. It contains Th to Lr.
Advantages of this kind of classification. As a result of this classification of elements were placed in correct positions in the periodic table. It shows a gradual gradation in physical and chemical properties of elements. The metallic nature gradually decreases and non – metallic nature
gradually increases from ‘s’ block to ‘p’ block. This classification gave a special place for radioactive elements.
Question 21.
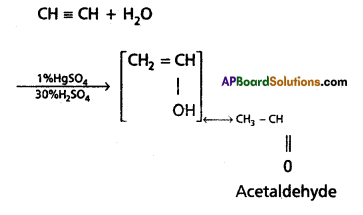
Give two methods of preparation of acetylene. How does it react with water and ozone ?
Answer:
Preparation of acetylene :
1. Dehydrohalogenation :
Acetylene is obtained when dibromo ethane is heated with alcoholic KOH.
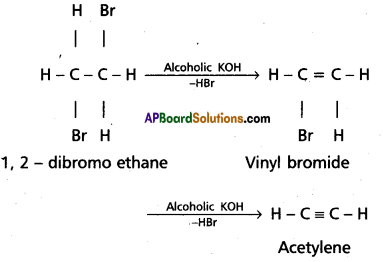
2. Acetylene is obtained by heating iodoform with silver powder.
![]()
3. Addition of water : Acetylene undergoes addition reaction with water in the presence of mercuric sulphate and sulphuric acid. Vinyl alcohol formed in the reaction undergoes rearra¬ngement and gives acetaldehyde.