Access to a variety of AP Inter 1st Year Chemistry Model Papers and AP Inter 1st Year Chemistry Question Paper March 2019 helps students overcome exam anxiety by fostering familiarity. question patterns.
AP Inter 1st Year Chemistry Question Paper March 2019
Note : Read the following instructions carefully.
- Answer all questions of Section ‘A’. Answer any six questions in Section ‘B’ and any two questions in Section ‘C’.
- In Section ‘A’, questions from Sr. Nos. 1 to 10 are of “Very Short Answer Type”. Each question carries two marks. Every answer may be limited to 2 or 3 sentences. Answer all these questions at one place in the same order.
- In Section ‘B’, questions from Sr. Nos. 11 to 18 are of “Short Answer Type”. Each question carries four marks. Every answer may be limited to 75 words.
- In Section ‘C’, questions from Sr. Nos. 19 to 21 are of “Long Answer Type”. Each question carries eight marks. Every answer may be limited to 300 words.
- Draw labelled diagrams wherever necessary for questions in Sections ‘3’ and ‘C’.
Section – A
Note : Answer all questions.
Question 1.
What is Plaster of Pgris? Write its uses.
Answer:
Plaster of paris is the hemi hydrate of CaSO4 with formula
CaSO4. \(\frac{1}{2}\)H2O.
Preparation :
Plaster of paris is obtained by heating gypsum at 393 K.
![]()
- If temperature is used greater than 393 K then an hydrous CaSO2 is formed which is called ‘dead burnt plaster’.
- Plaster of paris has an important property of setting with water.
- It forms a hard solid in 5 to 15 min. when it is mixed with suit¬able quantity of water.
It is majorly used in building industry and as well as plasters. - It is used in the bone fractures (or) sprain conditions. It is used in dentistry. It is used in manufacturing status and busts.
Question 2.
What Agrochemicals are responsible for water pollution?
Answer:
Agrochemicals like chemical fertilisers, chemicals used for killing insects, fungi and weeds in crop etc., are responsible for water pollution.
Question 3.
Name the common components of photo chemical smog.
Answer:
The common components of Photo Chemical smog are O3, NO, acrolein, formaldehyde and Peroxy Acetyl Nitrate (PAN).
Question 4.
Potassium carbonate cannot be prepared by Solvay process. Why?
Answer:
Potassium carbonate cannot be prepared by solvay process because potassium bi carbonate is more soluble and to be precipitated by the addition of ammonium bi carbonate to a saturated solution of potassium chloride.
Question 5.
What is the effect of pressure on a gaseous chemical equilibrium?
Answer:
Increase of external pressure of the reaction at equilibrium favours the reaction in the direction in which the volume (or) the No. of molecules decreases. Decrease of external pressure of reaction at equilibrium favour the reaction in the direction in which the volume (or) no. of molecules increase.
Question 6.
What are Extensive and Intensive properties?
Answer:
Measurable (or) macroscopic properties such as mass, pressure, volume, temperature, surface tension, viscosity etc., can be subdivided into two categories as below :
i) Extensive properties:
The properties whose magnitude depends upon the quantity of matter present in the system are called extensive properties. Examples of such properties are mass, volume, heat capacity, internal energy, entropy, heat content, Gibbs free energy etc. These properties change with quantity of matter present in the system. These properties are additive in nature.
ii) Intensive properties :
The properties which do not depend upon the quantity of matter present in the system are called intensive properties. Examples of such properties are density, mojar volume, molar entropy, molar heat capacity, surface tension, viscosity, specific heat, refractive index, pressure, temperature, boiling point, freezing point, vapour pressure etc. These properties depends only on the nature of the substance.
Question 7.
State the 3rd law of thermo dynamics.
Answer:
Third law of thermodynamics : ‘The entropy of a pure and perfectly crystalline substance is zero at the absolute zero of a temperature (- 273° C).
Slim T → 0 = 0
This law imposes a limitation on the value of entropy.

Question 8.
Calculate the amount of Carbon dioxide that could be produced when one mole of Carbon is burnt in 16g of dioxygen.
Answer:
C + O2 → CO2
12g.32g → 44g
16g → 22g
Question 9.
Calculate the ratio of kinetic energies of 3g of H2 and 4g of O2 at a given temperature.
Answer:
Given 3g of H2 and 4g of O2
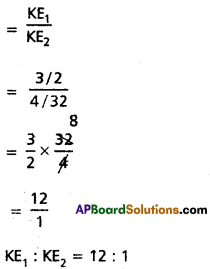
Question 10.
Write IUPAC names of the following compounds :
a) (CH3)2 C(C2H5)2
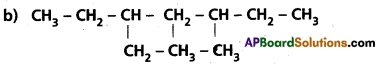
Answer:
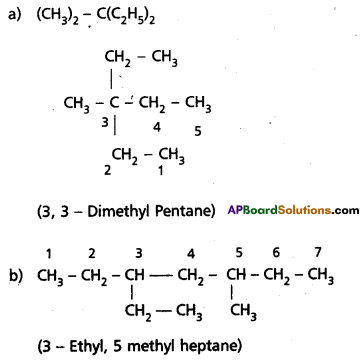
Question 11.
Deduce : (a) Charle’s law (b) Graham’s law of diffusion from kinetic gas equation.
Answer:
a) Deduction of Charle’s law :
Kinetic gas equation is PV = \(\frac{1}{3}\) mnu2
PV = \(\frac{1}{3}\)mnu2 = \(\frac{2}{2} \times \frac{1}{3}\)mnu2
= \(\frac{2}{3} \times \frac{1}{2}\) mnu2 = \(\frac{2}{3}\)(KE)
[Kinetic energe (KE) = \(\frac{1}{2}\)mnu2
PV = \(\frac{2}{3}\)KT
[According to kinetic theory KE = KT]
\(\frac{V}{T}=\frac{2}{3} \times \frac{K}{P}\)
∴ \(\frac{V}{T}\) (or) V ∝ T
Hence Charle’s law proved from kinetic gas equation.
b) Graham’s law : At constant temperature and pressure the rate of diffusion of a gas is inversely proportional to the square root of its density r ∝ \(\frac{1}{\sqrt{d}}\)
Deduction : Kinetic gas equation is
PV = \(\frac{1}{3}\) mnu2 = \(\frac{1}{3}\)Mu2
u2 = \(\frac{3 P V}{M}=\frac{3 P}{d}\)
∴ u = \(\sqrt{\frac{3 P}{d}}\)
At constant pressure u = k. \(\frac{1}{\sqrt{d}}\)
k = constant
or u ∝ Vd \(\frac{1}{\sqrt{d}}\)
∵ The rate of diffusion of gases depends upon the velocity of the gas molecules.
So r ∝ \(\frac{1}{\sqrt{d}}\)
This is Graham’s law.
Question 12.
Balance the following redox reaction in basic medium by ion- electron method :
MNO4(aq)– + I(aq)– → MnO2(s) + ls(S)
Answer:
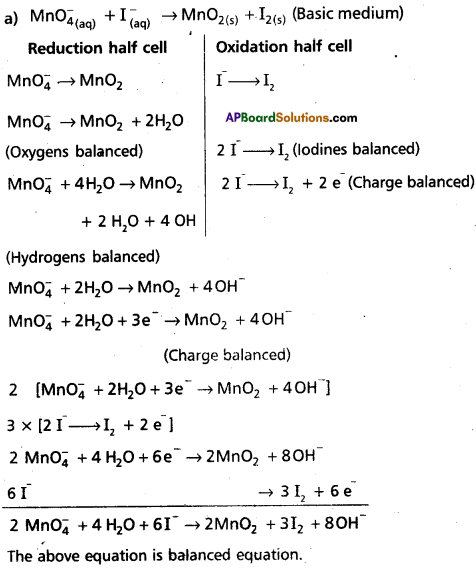
Question 13.
What is a conjugatic acid-base pair? Write the conjugate acid and conjugate base of each of the following :
a) OH–
b) HCO3
Answer:
a) OH– + H+ → H2O
Proton accepto Hence a Bronsted base.
b) HCO3
HCO3 → H2CO3
Question 14.
Explain the following with suitable examples :
a) Electron deficient hydrides
b) Ionic hydrides
Answer:
a) Electron – deficient hydrides:
These are the molecular hydrides in which the available no.of valency electrons is less than the number required for normal covalent bond formation. These are the molecular hydrides in which the available valency elec¬trons are less than the required for writting the Lewis structure of the molecule. Eg : (AlH3)n, B2H6 etc.
These hydrides acts as Lewis acids i.e. electron pair acceptors. These forms dative bond with donors,
b) Ionic hydrides :
- These are also called as saline hydrides (or) salt like hydrides. These are the hydrides formed by combining di hydrogen with s-block elements (Electro positive elements).
- These are stoichiometric compounds.
Eg : LiH, NaH, CaH2 etc. - NaH is formed by the direct union of Na and H2.
![]()
Question 15.
Explain the structure of the diborane.
Answer:
Diborane is an electron deficient compound. It has ‘ 12 ‘ valency electrons for bonding purpose instead of ’14’ electrons. In diborane each boron atom undergoes sp3 hybridization out of the four hybrid orbitals one is vacant.
Each boron forms two, σ – bonds ( 2 centred – 2 electron bonds) bonds with two hydrogen atoms by overlapping with their ‘1s’ orbital. The remaining hybrid orbitals of boran used for the formation of B bridge bonds.
In the formation of B – H – B bridge, half filled sp3 hybrid ofbital of one boron atom and vacant sp3 hybrid orbital of second boron atom overlap with 1s orbital of H – atom.
These three centred two electron bonds are also called as banana bonds. These bonds are present above and below the plane of BH2 units. Diborane contains two coplanar BH2 groups. The four hydrogen atoms are called terminal hydrogen atoms and the remaining two hydrogens are called bridge hydrogen atoms.
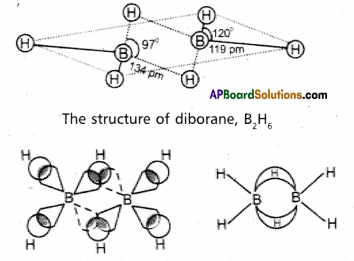
Bonding in diborane, Each B atom uses sp3 hybrids for bonding. Out of the four sp3 hybrids on each B atom, one is without an electron shown in broken lines. The terminal B-H bonds are normal 2-centre-2-electron bonds but the two bridge bonds are 3-centre-2-electron bonds. The 3-centre-2electron bridge bonds are also referred to as banana bonds.
Question 16.
What do you understand by
a) Allotropy
b) Inert pair effect.
Answer:
a) Allotropy :
- The phenomenon of existence of an element in different physi¬cal forms having similar (or) same chemical properties is called allotropy.
- Crystalline allotropes of carbon are a) Diamond b) Graphite,
b) Inert pair effect:
The reluctance of ‘ns1 pair of electrons to take part in bond formation is known as inert pair effect.
(or)
The occurrence of oxidation states two units less than the group oxidation states is known as inert pair effect. Eg : Lead exhibits +2 oxidation state as stable oxidation state due to inert pair effect. (Instead of +4 state).
Question 17.
Describe any two methods of preparation of Ethane.
Answer:
Methods of preparation of ethane :
1. Decarboxylation :
Ethane is prepared by heating sodium propionate with sodalime.
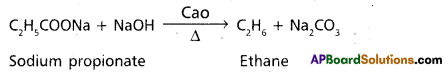
2. Kolbe’s electrolysis :
Ethane is obtained by the electrolysis of potassium acetate solution.
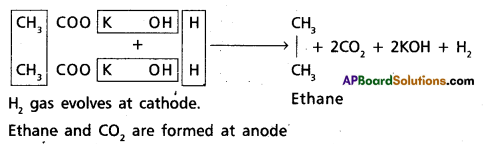
Question 18.
Write the reactions of Ethylene with the following :
a) Ozone
b) Cold, dilute alk. KMnO4.
Answer:
a) With O3:
Ethylene undergoes addition reaction with ozone and gives a cyclic compound called ozonide. It undergoes hydrolysis in the presence of zinc dust to give formaldehyde.
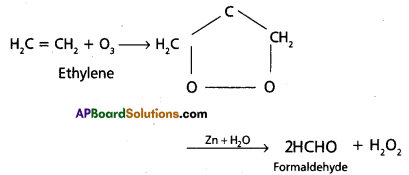
c) Cold and dil. alk. KMnO4:
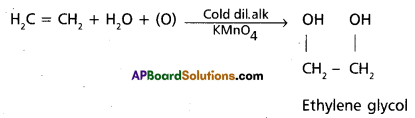
Pink coloured cold and dil. alkaline KMn04 solution is Bayer’s reagent. Ethylene decolourises Bayers reagent to give ethylene glycol. This is test for unsaturation.
Question 19.
a) What are postulates of Bohr’s model of Hydrogen atom?
b) State Hund’s rule and Aufbau principle.
Answer:
a) Refer Q.No. 19(a) in T.S May – 2018 Paper.
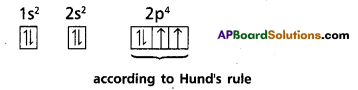
b) Hund’s rule : This rule deals with the filling of electrons in degenerate orbitals. It states “Pairing of electrons in the orbitals belong¬ing to the same subshell (p, d or f) does not take place until each orbital belonging to that subshell has got one electron each (i.e.,) all the orbitals are singly occupied”.
Since there are three ‘p’, five ‘d‘ and seven f orbitals, therefore the pairing of electrons will start in the p, d and f orbitals with the entry of 4th, 6th and 8th electrons respectively.
Ex : ‘8O’ electronic configuration is
Aufbau’s principle : This principle states :
“In the ground state of the “In the ground state of the atoms, the orbitals are filled in order of their increasing energies”. In other words electrons first occupy the lowest energy orbital available to them and enter into higher energy orbitals only after the lower energy orbitals are filled.
The order in which the orbitals are filled as follows :
1s < 2s < 3s < 3P < 4s < 3d < 4p < 5s < 4d < 5p < 4f < 5d < 6p < 7s
Question 20.
Write an essay on s. p, d and f-block elements.
Answer:
Write an essay on s, p, d and f-block elements.
Answer:
According to the electronic configuration of elements, the elements have been classified into four blocks. The basis for this classification is the entry of the differentiating electron into the subshell. They are classified into s, p, d and f blocks.
‘s’ block elements:
If the differentiating electron enters into ‘s’ orbital, the elements belongs to ‘s’ block. In every group there are two ‘s’ block elements.
As an ‘s’ orbital can have a maximum of two electrons, ‘s’ block has two groups IA and IIA.
‘p’ block elements:
If the differentiating electron enters into ‘p’ orbital, the elements belongs to’p’block. ‘p’ block contains six elements in each period. They are IIIA to VII A and zero group elements. The electronic configuration of ‘p’ block elements varies from ns2np1 to ns2np6.
‘d’ block elements: It the differentiating electron enters into (n – 1) d – orbitals the elements belongs to’d’ block. These elements are in between ‘s’ and ‘p’ blocks. These elements are also known as transition elements.
In these elements n and (n – 1) shells are incompletely filled. The general electronic configuration of’d’ block elements is (n – 1) d1-10 ns1-2 . This block consists of IIIB to VIIB, VIII, IB and IIB groups.
‘f’ block elements: If the differentiating electron enters into ‘f’ orbitals of antipenultimate shell (n – 2) of atoms of the elements belongs to ‘f’ block. They are in sixth and seventh periods in the form of two series with 14 elements each. They are known as lanthanides and actinides and are arranged at the bottom of the periodic table. The general electronic configuration is (n – 2) f1-14 (n-1) d0-1 ns2
In these shells the last three shells (ultimate, penultimate and anti penultimate) are incompletely filled. Lanthanides belongs to 4f series. It contains Ce to Lu. Actinides belong to 5f series. It contains Th to Lr.
Advantages of this kind of classification :
As a result of this classification of elements were placed in correct positions in the periodic table. It shows a gradual gradation in physical and chemical properties of elements. The metallic nature gradually decreases and non – metallic nature gradually increases from’s” block to ‘p’ block. This classification gave a special place for radioactive elements.
Question 21.
a) Explain the hybridisation involved in SFg.
b) State Fajan’s Rules and give suitable examples.
Answer:
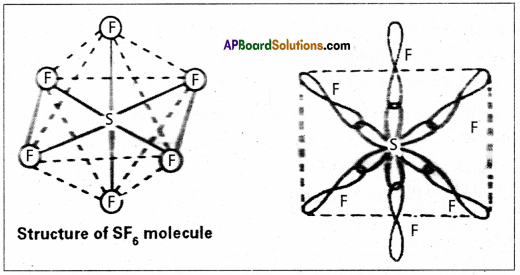
a) In this hybridisation one ‘s’ orbital, three ‘p’ orbitals and two’d’ orbitals of the excited atom combine to form six equivalent sp3d2 hybrid orbitals, e.g. : SF6
These six sp3 d2 hybrid orbitals overlap overlap six 2pz orbitals of fluorine atoms to form six σsp3d2 bonds. The directions of the bonds give an octahedral shape to the molecule. The bond angle is 90° or 180° & 90°.
b) Fajan’s rules :
1) Ionic nature of the bond increases with increase in the size of cation, e.g. : The ionic nature increases in the order
Li+ < Na+ < K+ < Rb– < Cs+
2) The formation of ionic bond is favoured with the decrease of the size of anion.
e.g. : CaF2 is more ionic than Cal2.
3) If the charge on cation (or) anion (or) both is less, then they can form ionic bonds.
e.g.: The ionic nature increases in the order.
AlCl3 < MgCl2 < NaCl
Al3+ Mg2+ Na+.