Access to a variety of AP Inter 1st Year Chemistry Model Papers and AP Inter 1st Year Chemistry Question Paper March 2018 helps students overcome exam anxiety by fostering familiarity. question patterns.
AP Inter 1st Year Chemistry Question Paper March 2018
Note : Read the following instructions carefully.
- Answer all questions of Section ‘A’. Answer any six questions in Section ‘B’ and any two questions in Section ‘C’.
- In Section ‘A’, questions from Sr. Nos. 1 to 10 are of “Very Short Answer Type”. Each question carries two marks. Every answer may be limited to 2 or 3 sentences. Answer all these questions at one place in the same order.
- In Section ‘B’, questions from Sr. Nos. 11 to 18 are of “Short Answer Type”. Each question carries four marks. Every answer may be limited to 75 words.
- In Section ‘C’, questions from Sr. Nos. 19 to 21 are of “Long Answer Type”. Each question carries eight marks. Every answer may be limited to 300 words.
- Draw labelled diagrams wherever necessary for questions in Sections ‘3’ and ‘C’.
Section – A
Note : Answer all questions.
Question 1.
Write two adverse effects caused by acid rains.
Answer:
Effects of acid rains :
- Acid rains are harmful for agriculture, trees and plants because it dissolves and washes away nutrients needed for their growth.
- Acid rains affects the plants and animal life in aquatic eco-system.
- Acid rains damages the old buildings and historical monuments like Taj mahal.
- Acid rains corrodes water pipes which lead to decrease the quality of drinking water.
Question 2.
Define the terms ‘Receptor’ and ‘Sink’.
Answer:
Sinks: The medium which retains and interacts with long level pollutant is called sink.
Receptor: The medium which is effected by a pollutant is called receptor.
![]()
Question 3.
What is surface tension?
Answer:
The force acting at right angles to the surface of the liquid along unit length of surface is called surface tension.
Units : dynes / cm.
Question 4.
4 grams of NaOH dissolved in 250 ml of the solution. Calculate Molarity.
Answer:
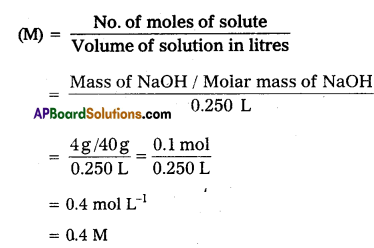
Question 5.
Write the conjugate acid and conjugate base of each of the following :
(a) HSO4–
(b) H2O
Answer:
| Species | Conjugate acid | Conjugae base |
| (a) HSO4– | H2SO4 | SO42- |
| (b) H2O | H2O+ | OH2- |
Question 6.
Give the biological importance of Na+ and K+ ions.
Answer:
- Na+ ions participate in the transmission of nerve signals.
- Na+ ions regulates the flow of water accross cell membranes.
- Na+ ions responsible for transport of sugars and amino acids into cells.
- K+ ions Eire useful in activating enzymes.
- K+ ions participate in the oxidation of glucose to produce ATE
- K+ along with Na+ responsible for the transmission of nerve signals.
![]()
Question 7.
What is Plaster of Paris? Write its uses.
Answer:
Plaster of paris is the hemi hydrate of CaSO4 with formula CaSO4.\(\frac{1}{2}\)H2O.
Uses:
- It is majorly used in building industry and as well as plasters.
- It is used in the bone fractures (or) sprain conditions.
- It is used in dentistry.
- It is used in manufacturing status and busts.
Question 8.
Why is ‘CO’ poisonous?
Answer:
- ‘CO’ gas is highly poisonous because it has the ability to form a stable complex with haemoglobin.
Hb + CO → Hb – CO Haemoglobin Carboxy haemoglobin (stable complex) - Carboxy haemoglobin is 300 times more stable than oxyhae- moglobin.
Question 9.
How is water gas prepared?
Answer:
Water gas is prepared by passing superheated steam over hot coke.

Question 10.
Write IUPAC names of the following compounds :

Answer:
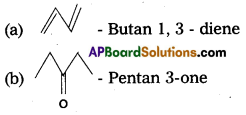
Section – B
Question 11.
Explain different types of hydrogen bonds with examples.
Answer:
Hydrogen bond is a weak electrostatic bond formed between partially positive charged hydrogen atom and an highly electronega¬tive atom of the same molecule or another molecule. Hydrogen bond is formed when the Hydrogen is bonded to small, highly electronegative atoms like F, O and N. A partial positive charge will be on hydrogen atom and partial negative charge on the electronegative atom.
The bond dissociation energy of hydrogen bond is 40 KJ/mole. Hydrogen bond is represented with dotted lines (…….). Hydrogen bond is stronger than Vander Waals’ forces and weaker than covalent bond.
Hydrogen bonding is of two types. (1) Intermolecular hydrogen bond and (2) Intramolecular hydrogen bond.
1) Intermolecular hydrogen bond :
If the hydrogen bond is formed between two polar molecules it is called intermolecular hydrogen bond, i.e., the hydrogen bond is formed between hydrogen atom of one molecule and highly electronegative atom of another molecule is known as intermolecular hydrogen bond.
Ex.: Water (H2O) ; HF : NH3; p – nitrophenol, CH3COOH, ethyl alcohol etc.

Water molecule forms an associated molecule through intermolecular hydrogen bond. Due to molecular association water possess high boiling point.
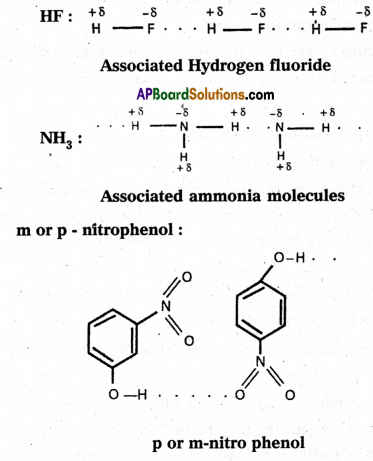
2) Intramolecular hydrogen bond :
If the hydrogen bond is formed within the molecule it is known as intramolecular hydrogen bond.
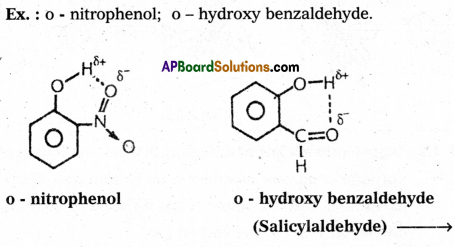
Question 12.
What is Hybridization? Explain the structure of CH4 on the basis of Hybridization.
Answer:
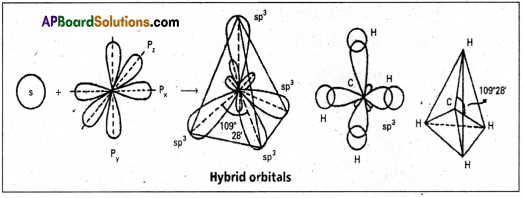
Hybridisation : The inter mixing of atomic orbitals of an atom of identical energy to form equal no. of new orbitals is called hybridisation.
Formation of Methane molecule :
- The central atom of methane is carbon.
- The electronic configuration of carbon in ground state is Is2 2s2 2p2x 2py2 2pz0° and on excitation it is 1s2 2s1 2px1 2py1 2pz1. During excitation the 2s pair splits and the electron jumps into the adjacent vacant 2pz orbital.
- The 2s1 2px1 2py1 2pz1 undergo sp3 hybridisation giving four equivalent sp3 hybridised orbitals.
- Each sp3 hybrid orbital overlaps with the Is orbitals of hydrogen forming σsp3 – s bond.
- In case of methane four σsp3 – s bonds are formed. The bonds are directed towards the four comers of a regular tetrahedron. The shape of methane molecule is tetrahedral with a bond angle 109°28′.
Question 13.
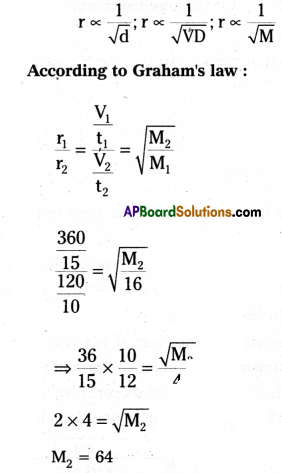
State Graham’s law of diffusion. 360 cm3 methane gas diffused through a porous membrane in 15 minutes. Under similar conditions 120 cm3 another gas diffused in 10 minutes. Find the molar mass of the second gas.
Answer:
Graham’s law of diffusion:
At a given temperature and pres-sure, the rate of diffusion of a gas is inversely proportional to the square root of density, vapour density or molecular weight.
Question 14.
Balance the following Redox reaction by Ion-electron method in acidic medium.
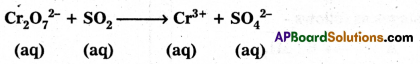
Answer:
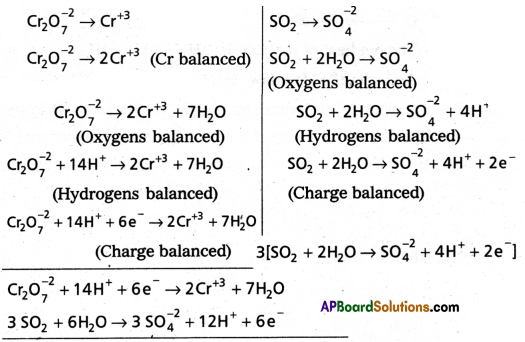
Question 15.
State and explain the Hess law of constant heat summation with an example.
Answer:
Hess’s law states that the total amount of heat evolved or ab-sorbed in a chemical reaction is always same whether the reaction is carried out in one step (or) in several steps.
Illustration : This means that the heat of reaction depends only on the initial and final stages and not on the intermediate stages through which the reaction is carried out. Let us consider a reaction in which A gives D. The reaction is brought out in one step and let the heat of reaction be ΔH.
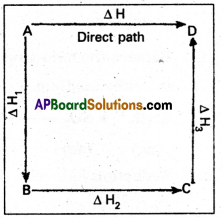
A → D; ΔH
Suppose the same reac-tion is brought out in three stages as follows
A → B : ΔH1
B → C : ΔH2
C → D : ΔH3
Img-1
The net heat of reaction is ΔH1 + ΔH2+ ΔH3.
According to Hess law AH = ΔH1 + ΔH2+ ΔH3.
Ex : Consider the formation of C02. It can be prepared in two ways.
1) Direct method: By heating carbon in excess of O2.
C(s) + O2(g) → CO2(g); ΔH = – 393.5 kJ
2) Indirect method : Carbon can be converted into CO2 in the following two steps.
C(s) + \(\frac{1}{2}\) O2(g) → CO2(g); ΔH1 = – 110.5 kJ
CO (g) + \(\frac{1}{2}\) O2(g) → CO2(g); ΔH2 = – 283.02 kJ
Total ΔH = -393.52 kJ (ΔH1 + ΔH2)
The two ΔH values are same.
![]()
Question 16.
Discuss the application of Le Chatlier’s principle for the industrial synthesis of Ammonia by Haber’s process.
Answer:
Applications of Le Chatelier’s principle to synthesis of Ammonia by Haber’s process :
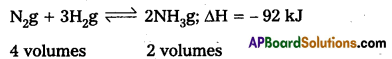
Nitrogen and Hydrogen combine to form ammonia. The formation of ammonia is reversible and exothermic reaction. It is accompanied by decrease in volume.
Effect of Pressure:
1 volume of N2 combines with 3 volumes of H2 to form 2 volumes of NH3. There is decrease in volume in the forward reaction (4 volumes to 2 volumes). According to Le Chatlier’s principle increase of pressure favours the reaction where there is decrease in volume. So higher the pressure, greater the yield of ammonia. In practice 200 atmospheres are used in the manufacture of ammonia by Haber’s process. Low pressures favour the reverse reaction i.e., decomposition of NH3 already formed.
Effect of Temperature :
The formation of ammonia (forward reaction) is exothermic reaction N2 + 3H2 → 2 NH3; ΔH = – 92 kJ. Low temperatures favour the forward reaction. But at low temperatures the reaction is too slow. Therefore an optimum tempera¬ture (725 K – 775 K) is chosen in Haber’s process. To speed up the reaction,-a catalyst, finely divided iron is used. To increase the activity of the catalyst molybdenum or a mixture of oxides of K and Al is used as promoter.
The reverse reaction (i.e.,) decomposition of NH3 is an endothermic reaction. High temperatures favour the decomposition of NH3. Therefore high temperatures are avoided in Haber’s process.
Thus the optimum conditions are
Pressure : 200 atm
Temperature : 725 – 775 K
Catalyst : Fe (Powered)
Promoter : Mo (or) (K2O + Al2O3)
Question 17.
Explain the terms ‘Hard water’ and ‘Soft water1. Write a note
on ‘Calgon method’ for the removal of hardness of water.
Answer:
Hard water : Water does not give lather readily with soap is called hard water.
- Hard water contains hardness. This hardness is due to presence of Ca, Mg soluble salts.
- Presence of Ca, Mg – bicarbonates causes temporary hardness.
- Presence of Ca, Mg – chlorides, sulphates causes permanent hardness.
Soft water : Water which give lather immediately with soap is called soft water.
(or)
Water which is free from soluble salts of Ca, Mg is called soft water.
Calgon process :
- Calgon is sodium hexametaphosphate. [Na6P6O18 (or) (NaPO36j
- Calgon doesnot precipitate the Ca (or) Mg – salts but re¬moves Ca+2 and Mg+2 ions from water.
- The removal of Ca+2 (or) Mg+2 ions from water may takes place either by adsorption (or) by complex formation.
Reactions:
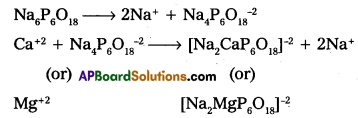
Question 18.
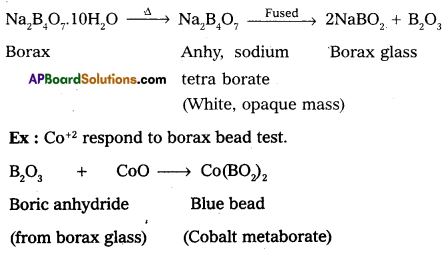
Explain Borax bead test with suitable example.
Answer:
Borax bead test: This test is useful in the identification of basic radicals in qualitative analysis. On heating borax swells into a white, opaque mass of anhydrous sodium tetra borate. When it is fused, borax glass is obtained. Borax glass is sodium meta borate and B6O3. The boric anhydride, B2O3, combined with metal oxides to form metal metaborates as coloured beads. The reactions are as follows :
Section – C
Question 19.
Write the postulates of Bohr’s model of hydrogen atom. Discuss the importance of this model to explain various series of line spectra in hydrogen atom.
Answer:
Niels Bohr quantitatively gave the general features of hydrogen atom structure and it’s spectrum. His theory is used to evaluate several points in the atomic structure and spectra. The postulates of Bohr atomic model for hydrogen as follows.
Postulates:
- The electron in the hydrogen atom can revolve around the nucleus in a circular path of fixed radius and energy. These paths are called orbits (or) stationary states. These circular orbits are concentric (having same center) around the nucleus.
- The energy of an electron in the orbit does not change with time. When an electron moves from lower stationary state to higher stationary state absorption of energy takes place.
- When an electron moves from higher stationary state to lower stationary state emission of energy takes place.
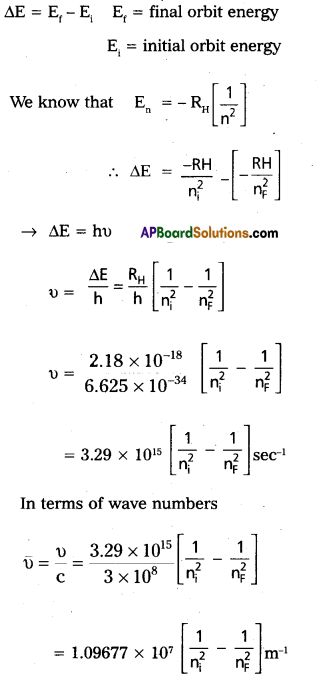
- When an electronic transition takes place between two stationary states that differ in energy by ∆E is given by ∆E = E2 – E1 = hν
∴ The frequency of radiation absorbed (or) emitted ν = \(\frac{E_2-E_1}{h}\) E1
and E2 are energies of lower, higher energy states respctiveiy.
The angular momentum of an electron is given by mvr = \(\frac{\mathrm{nh}}{2 \pi}\)
An electron revolve only in the orbits for which it’s angular momentum is integral multiple of \(\frac{h}{2 \pi}\).
Line spectra of Hydrogen – Bohr’s Theory :
1. In case of hydrogen atom line spectrum is observed and this can be explained by using Bohr’s Theory.
2. According to Bohr’s postulate when an electronic transition takes place between two stationary states that differ in energy is given by
3. In case of absorption spectrum nf > ni → energy is absorbed (+Ve).
4. In case of emission spectrum ninf → energy is emitted (-Ve).
5. Each spectral line in absorption (or) emission spectrum as¬sociated to the particular transition in hydrogen atom.
6. In case of large no.of hydrogen atoms large no.of transitions possible they results in large no.of spectral lines.
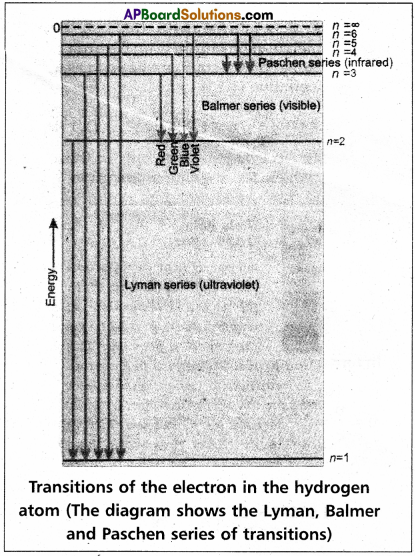
The series of lines observed in hydrogen spectra are
| ‘n’ value | Series | Region |
| 1 | Lyman series | UV region |
| 2 | Balmer series | visible |
| 3 | Paschen series | Near I.R. |
| 4 | Brackette series | I.R. |
| 5 | Pfund series | Far I.R. |
Question 20.
What is periodic property? How the following properties vary in a group and in a period? Explain.
(a) Ionization Enthalpy
(b) Electro-negativity
(c) Metallic character.
Answer:
Periodic Property : The property which change gradually with change in electronic configuration and repeats in regular intervals is called periodic property.
a) Ionization Enthalpy :
In groups and periods of the periodic table the ionization enthalpy values are periodically change depend upon the electronic configuration and size of elements.
In a group of elements ionization energy decreases from top to bottom because atomic radius increases.
In general, in a period the atomic size decreases. Because of this, the ionization energy increases across a period.
b) Electronegativity :
Electronegativity increases from left to right in a period since the atomic: radii decreases and nuclear attraction increases.
In a group electronegativity decreases from top to bottom due to an increase in atomic radii and a decrease in nuclear attraction.
c) Metallic character :
In a group metallic character increase from top to bottom due to increase of atomic size in a periodic metallic haracter decreases from left to right due to decrease of atomitic size.
![]()
Question 21.
Describe two methods of preparation of Benzene. Explain Halogenation and Alkylation reactions of Benzene.
Answer:
Preparation of Benzene :
1. When sodium benzoate distilled with sodalime. Benzene is formed.
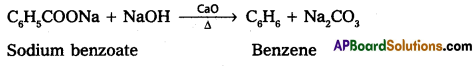
2. Acetaylene undergo polymerisation to from Benzene.
![]()
(l)Halogenation: Benzene reacts with chlorine in the presence of FeCl3 or AlCl3
to give chloro-benzene.
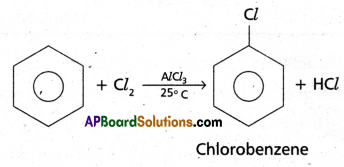
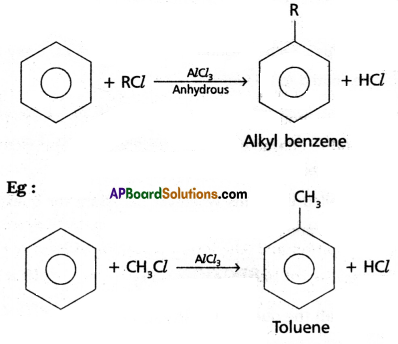
(2)Friedel – Craft’s Alkylation :
Benzene reacts with alkyl halides in the presence of AlCl3 to give alkyl benzene.