Students get through AP Inter 1st Year Chemistry Important Questions 13th Lesson Organic Chemistry-Some Basic Principles and Techniques which are most likely to be asked in the exam.
AP Inter 1st Year Chemistry Important Questions 13th Lesson Organic Chemistry-Some Basic Principles and Techniques
Very Short Answer Questions
Question 1.
Write the reagents required for conversation of benzene to methyl benzene.
Answer:

Therefore, the required reagents are methyl chloride and anhydrous AlCl3.
This reaction is known as Friedel Craft’s alkylation.
Question 2.
How is nitrobenzene prepared? [AP 15]
Answer:
Benzene on heating with the mixture of cone. HNO3 & cone. H2SO4 at 60°C forms Nitro benzene.

Question 3.
Write the conformations of ethane.
Answer:
Conformational isomers are due to rotation along C-C single bond in alkanes.
Conformations of ethane:
Free rotation about C-C single bond in ethane gives an infinite number of confotmers. Out of these. Eclipsed and Staggered conformations are most significant.
Question 4.
How do you prepare ethyl chloride from ethylene?
Answer:
Ethylene combines with hydrogen chloride to form ethyl chloride.
CH2 = CH22 + HCl → CH3 – CH2Cl
Question 5.
Write HJPAC names of: [AP, TS 16, 18][Mar’ 13]
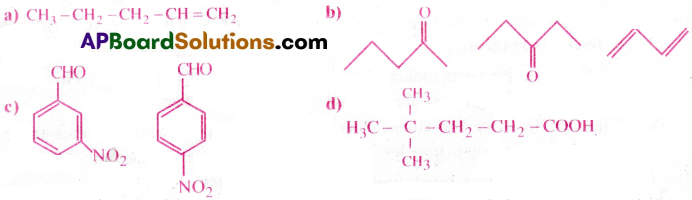
Answer:
a) 1 – Pentene
b) 2 -Pentanone, 3- Pentanone, 1,3-Butadiene
c) 3-nitro benzene carbaldehyde, 4-nitro benzene carbaldehyde
d) 4,4 Dimethyl pentanoic acid
![]()
Question 6.
Write the structures of Trichloroethanoic acid, Neopentane, p-nitrobenzaldehyde. [TS 22]
Answer:

Question 7.
Discuss Lassaigne’s test.
Answer:
Lassaigne’s test is used to detect nitrogen, sulphur, halogens and phosphorous in a given organic compound.
In Lassaigne’s test, the organic compound is mixed with a sodium metal and fused strongly.
If Nitrogen is present in the organic compound, it reacts with sodium & carbon resulting sodium cyanide.
If sulphur is present in the organic compound, it reacts with sodium resulting sodium sulphide.
It halogens are present in the organic compound, they result sodium halides.
Question 8.
Explain the principle of Chromatography. [Imp.Q]
Answer:
Chromatography is an important technique extensively used to separate mixtures into their components. Here, purification is based on adsorption (or) partition principle.
The mixture of substances is applied onto a stationary phase, which may be a solid or a liquid. A pure solvent or a mixture of solvents is allowed to move slowly over the stationary phase.
The components of the mixture get gradually separated from one another.
Question 9.
Explain why an organic liquid vaporizes at a temperature below its boiling point in its steam distillation?
Answer:
In steam distillation, the liquid boils when the sum of vapour pressures due to the organic liquid (P1) and that due to water (P2) becomes equal to the atmospheric pressure (P), i.e.,P = P1 + P2. Hence even though P1 is lower than P, the organic liquid vaporizes at lower temperature than its boiling point.
![]()
Question 10.
Explain the following (a) Crystallisation (h) Distillation
Answer:
a) Crystallisation :
This technique is used for the purification of solid organic compounds.
This method is based on the difference in the solubilities of the compound and the impurities in a suitable solvent.
The impure compound is dissovled in a solvent, in which it is partially soluble at room temperature and appreciably soluble at higher temperature.
The solution is heated to get a saturated solution further the solution is cooled to get pure organic compound crystals by filtration.
On repeating this process finally we get very pure compound.
b) Distillation :
Distillation is the process of converting a liquid into vapour on heating and then cooling the vapour back to liquid state.
This method is used to separate
- Volatile liquids from non-volatile impurities and
- The liquids having a sufficient difference in their boiling points (>40°C),
Liquids having different boiling points vapourises at different temperatures.
These vapours are cooled and the liquids formed are collected separately.
Short Answer Questions
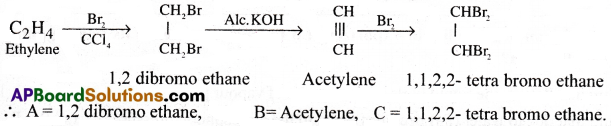
Question 1.
Write the corresponding equations for the following reactions and name the products A, B, C. [IPE’ 14][TS 15,16][AP I6]
![]()
Answer:

Question 2.
Name the products A, B, C formed in the following reactions, (live equations for the reactions. [Mar’09][TS 15]
![]()
Answer:
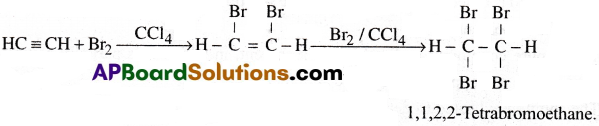
Question 3.
How does acetylene react with: (a) Bromine (b) Hydrogen? Write tile balanced equations for the above reactions. Name the products. [AP 16]
Answer:
a) Acetylene reacts with bromine in the presence of CCl4 and forms an addition compound 1, 1,2, 2-Tetra bromoethane.
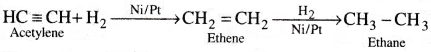
b) Hydrogen reacts with acetylene in the presence of Ni or Pt catalyst and forms ethane.
Question 4.
What is substitution reaction? Explain any three substitution reactions of Benzene. [May’ 10]
Answer:
Substitution reaction :
The reactions in which an atom or group present in a compound is replaced by another atom or group are known as substitution reactions.
The characterisitc reaction of benzene is Electrophilic substitution reaction.
Substitution reactions of benzene :
a) Benzene reacts with bromine or chlorine in the presence of AlCl3 and gives corresponding halo benzene, ie., Halogenation.
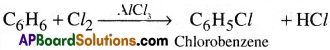
b) Benzene on heating with nitration mixture, below 60°C gives Nitrobenzene, ie., Nitration.
![]()
c) Benzene reacts with methyl chloride in the presence of anhydrous AlCl3 and gives Toluene. This reaction is known as Friedel crafts alkylation.

Question 5.
What is Dehydrohalogenation? Give the corresponding equation for the formation of alkene from alkyl halide.
Answer:
Dehydrohalogenation:
Removal of one hydrogen atom and one halogen atom from adjacent carbon atoms of a compound is called dehydrohalogenation.
Alkene from alkyl halide:
Ethyl Bromide on heating with ale. KOH undergoes dehydrohalogenation and gives ethylene.
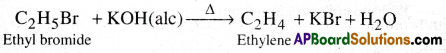
![]()
Question 6.
Which type of compounds react with Ozone? Explain with one example. [IPE’ 14]
Answer:
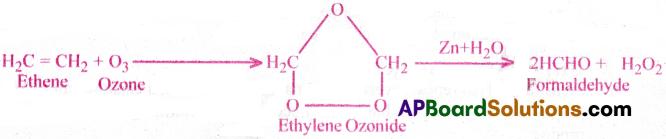
Unsaturated hydrocarbons usally react with ozone.
Ozone reacts with unsaturated hydrocarbons to form addition compounds called ozonides.
On hydrolysis the ozonides give carbonyl compounds. This process is called ozonolysis. Ozonolysis is used for the location of the double bond or triple bond in unsaturated compounds like alkene, alkyne and benzene.
Ex: Ethylene undergoes addition reaction with ozone and forms ethylene ozonide.
It on hydrolysis in the presence of Zn dust gives formaldehyde and H2O2.
Question 7.
Give two examples each for position and functional isomerism. [TS 22][AP,TS 16]
Answer:
Position Isomerism is due to difference in the position of a substituent or functional group.

Functional isomerism is due to difference in the functional group in the molecules.

Question 8.
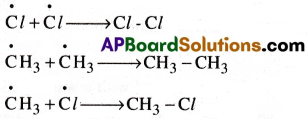
Explain the mechanism of halogenations of methane.
Answer:
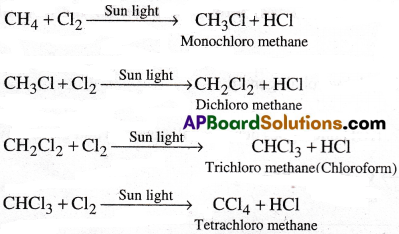
Halogenation:
Alkanes exhibit free radical substitution reactions.
Ex: Halogenation of methane: In the presence of sunlight or U.V.light, methane reacts with chlorine and undergoes substitution with chlorine atoms successively forming mono, di, tri and tetra chloro methane respectively.
Mechanism:
Halogenation of alkanes takes place by free radical mechanism. It involves three steps.
(i) Chain initiation:
In the presence of heat or light, chlorine molecule undergoes homolysis to form chlorine free radicals.
![]()
(ii) Chain propagation:
Chlorine free radical attacks methane molecule, breaking one of the C-H bonds and generating methyl free radical with the formation of HCl.
![]()
The methyl free radical thus obtained attacks second chlorine molecule to form CH3C/
with the liberation of another chlorine free radical.
![]()
Steps (a) and (b) repeat several times making the reaction a chain reaction. Many other propagation steps are possible and may occur, there by forming other chlorine substituted products.
(iii) Chain termination:
When two free radicals directly combine, the chain reaction terminates
Question 9.
How is ethylene prepared from ethyl alcohol? Write the reaction.
Answer:
Methods of preparation of Ethylene:
1) Dehydration of ethyl alcohol Ethyl alcohol on heating with con. H2SO4 at 170°C gives ethylene.
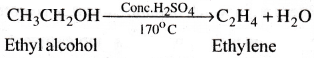
2) Dehydrohalogenation of ethyl halides:
Ethyl halides on heating with alcoholic NaOH or KOH gives ethylene.
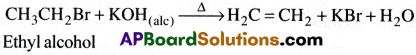
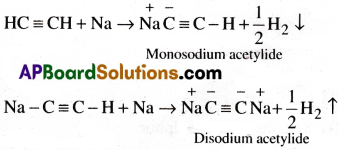
Question 10.
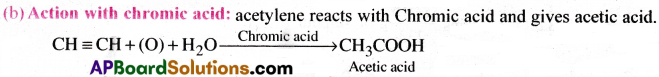
Explain the reactions of acetylene with (a) Na in NH3 (b) chromic acid:
Write the equations and name the products.
Answer:
(a) Action with Na in NH3:
Acetylene combines with sodium in liquid NH3 and gives mono sodium and disodium acetylides.
Question 11.
Explain crystallization and sublimation phenomena which are used in the puritlcation of organic compounds:
Answer:
a) Crystallisation:
This technique is used for the purification of solid organic compounds.
This method is based on the difference in the solubilities of the compound and the impurities in a suitable solvent.
The impure compound is dissovled in a solvent, in which it is partially soluble at room temperature and appreciably soluble at higher temperature.
The solution is heated to get a saturated solution further the solution is cooled to get pure organic compound crystals by filtration.
On repeating this process finally we get very pure compound.
b) Sublimation:
Sublimation is a purification method of solids.Some solid substances on heating directly pass into vapour state without melting. Those vapours on cooling form directly solid without condensing to liquid. This phenomenon is called sublimation.
Solid ⇌ Vapour
The compound to be purified is taken in a beaker covered with a watch glass and heated. The compound sublimes and solidifies on the lower surface of the watch glass. Impurities remain in the beaker. The compound is separated by scratching the watch glass.
![]()
Question 12.
Describe solvent extraction method to purify a compound.
Answer:
Solvent extraction:
It is used to separate an organic compound present in aqueous medium by using a solvent which is immisible with water and in which organic substance is more soluble. The aqueous solution of impure organic compound is shaken with a suitable solvent.
Organic compound goes into the organic solvent which is immisible with water.
The organic layer is separated and distilled to remove the liquid solvent.
The compound remains in distillation flask.
Question 13.
Explain the estimation of phosphorous and sulphur in the given organic compounds.
Answer:
Estimation of sulphur:
A known mass of the organic compound is heated with sodium peroxide in a Carius tube. Then ‘S’ is oxidised to H2SO4. The acid is precipitated as BaSO4 by adding excess of BaCl2 aq.solution. The ppt. is filtered, washed, dried and weighed.
Calculations:
Mass of organic compound = a g
Mass of barium sulphate = b g
Molecular weight of BaSO4 = 23
1 mole of BaSO4 or 233 g of BasO4 contains 32 g of sulphar.

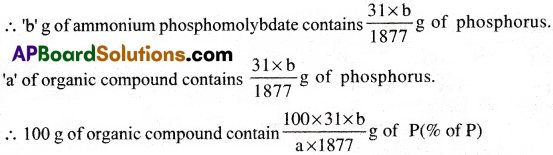
Estimation of phosphorous:
A known mass of organic compound is heated with fuming nitric acid in a Carius tube. Then phosphorus is oxidised to phosphoric acid. The acid is precipitated as ammonium phosphomolybdate by adding ammonia and ammonium molybdate solutions.
Calculations:
Mass of organic compound = a g
Mass of barium sulphate = b g
Molecular weight of ammonium phosphomolybdate = 1877
1877 g of ammonium phosphomolybdate contains 31 g of phosphorus
Question 14.
Explain addition of HBr to Propene with the ionic mechanism.
Answer:
Electrophilic addition of HBr to CH3-CH=CH2 molecule:
Addition of HBr to unsymmetrical alkene like propene generally follows Markownikoffs rule. According to this rule, the negative part of the reagent adds to the double bonded carbons bearing less number of hydrogens.
Mechanism:
1) HBr ionises to give H+ and Br (HBr → H+ + Br–)
2) Electrophile (H) attacks the double bond to form carbonium ion.
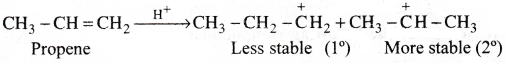
3) Nucleophile (Br–) attacks the carbonium ion to form 2-bromo propane as major product.

Question 15.
What is the product formed when sodium propionoate is heated with soda lime?
Answer:
When sodium propionoate is heated with soda lime, Ethane gas is obtained
![]()
This reaction is known as Decarboxylation. In these reactions the number of carbon atoms in the product is less than that of reactants ie., one carbon is less.
Long Answer Questions
Question 1.
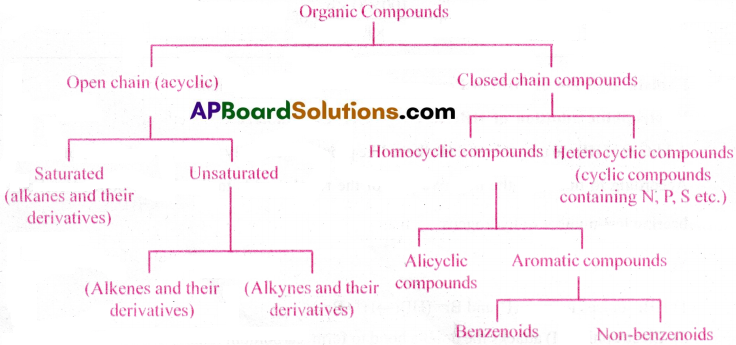
Explain the classification of hydrocarbons.
Answer:
Hydrocarbons are classified as open chain(acyclic/aliphatic) hydrocarbons and closed chain hydrocarbons. Aliphatic hydrocarbons are again classified as open chain hydrocarbons and closed-chain hydrocarbons i.e., Cyclo hydrocarbons.
Both open chain and closed chain hydrocarbons are again classified as hydrocarbons containing
i) C-C (alkanes and cycloalkanes)
ii) > C = C < (alkenes and cycloalkenes)
iii) – C≡C – (alkynes and cycloalkynes)
Question 2.
Write IUPAC names of the following compounds: [AP 26]
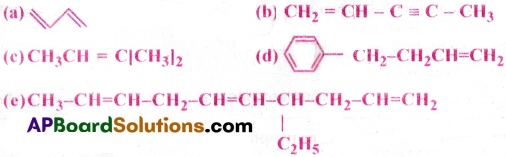
Answer:
(a) 1, 3-butadiene
(b) Pent-l-en-3-yne
(c) 2-methy 1-2-butene
(d) 4-pheny 1-1 -butene
(e) 4- Ethyl Deca-l,5,8-triene
![]()
Question 3.
a) Describe the methods of preparation of Ethane. [AP 19]
b) Explain the chemical properties of Ethane with equations. [TS 22]
Answer:
a) Preparation of ETHANE (C2H6) :
1) Decarboxylation:
Ethane is prepared by heating sodium propionate with sodalime (Sodalime is a mixture of NaOH & CaO)

2) Wurtz reaction :
Ethane is prepared by heating methyl iodide with sodium metal in the presence of dry ether. [AP 17][TS 15]
![]()
3) Kolbe’s electrolysis:
Ethane is obtained by the electrolysis of aqueous potassium acetate (or aqueous sodium acetate ). [TS 15]
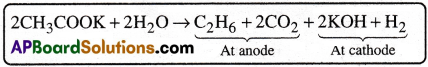
4) Sabatier – Senderen’s reduction reaction :
On a large scale, ethane is prepared by the catalytic hydrogenation of ethylene.
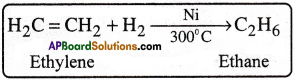
5) Reduction of ethyl iodide :
Ethane is prepared by the reduction of ethyl iodide in the presence of Zn and HCl.
![]()
b) Chemical Properties of Ethane:
1) Action with Chlorine (Chlorination):
Ethane reacts with chlorine in the presence of sunlight or UV light and gives ethyl chloride.

2) Action with Nitric acid(Nitration):
Ethane reacts with nitric acid vapours at 400°C and gives nitroethane.
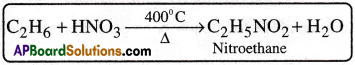
3) Action with Oxygen (Oxidation):
Ethane reacts with oxygen on combustion forms CO2 and H2O

Question 4.
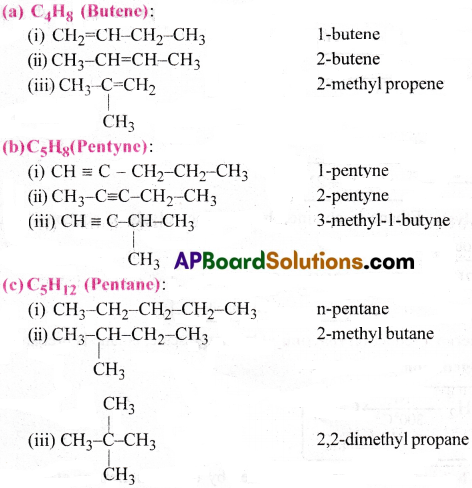
Write the structural formulas and IUPAC names for all possible isomers having the number of double or triple bond as indicated.
(a) C4H8 (one double bond)
(b) C5H8 (one triple bond)
(c) C5H12 (no multiple nonds)
Answer:
Question 5.
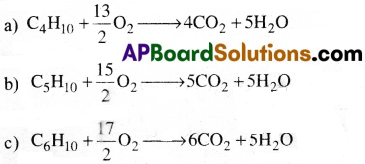
Write chemical equations for combustion reaction of the following hydrocarbons,
a) Butane b) Pentene c) Hexyne
Answer:
Question 6.
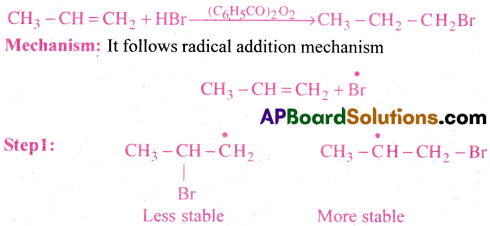
Addition HBr to propene yields 2-bromopropane, while in the presence of benzoyl peroxide, the same reaction yields 1-bromopropane. Explain and give mechanism.
Answer:
Addition of Hydrogen halides:
Addition of HX to a double bond generally follows Markownik off rule.
Statement of Markownikoff rule:
The rule states that addition of hydrogen halides to unsymmetrical alkenes takes place in such a manner that the positive part of the reagent attach itself to that carbon atom which has more number of hydrogen atoms.
Explanation:
It follows electrophilic addition mechanism.

Since 2° carbocation is more stable than l° carbocation, the major product formed is 2-Bromopropane.
Note: Markownikoff s rule is only applicable for unsymmetrical alkenes.
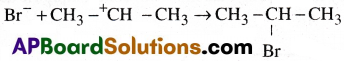
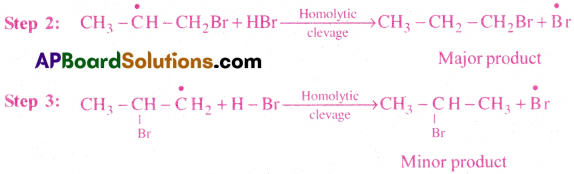
Anti Markovnikov’s rule (or) Kharasch effect:
In the presence of peroxide (R- O-O-R) the addition of hydrogen halides (HBr) to an unsymmetrical alkene like propene takesplace in such a way that the negative part of the reagent attach itself to that carbon atom which has more number of hydrogen atoms.
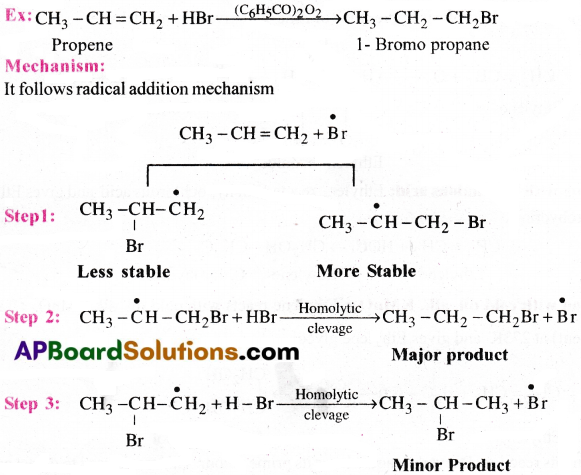
Stability of free radicals follows as 3°>2°>1°
Therefore, 1-bromopropane is major product.
Uses of Ethylene:
- It is used in the manufacture of ethyl alcohol, polythene etc.,
- In the preparation of ethylene glycol as antifreeze
- In the preparation of mustard gas.
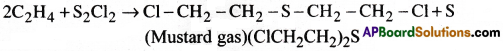
Question 7.
Describe two methods of preparation of ethylene. Give equation for the reactions of ethylene with the following. [TS 18][AP 16,19]
a) Ozone
b) llypohalous acid
c) Cold and dil.alk. KMnO4
d) Heated with O2 at high pressure
Answer:
a) preparation of Ethylene (C2H6):
1) Dehydration:
Ethylene is prepared by heating ethyl alcohol with cone, sulphuric acid at 170°C.
![]()
2) Dehydrohalogenation:
Ethylene is prepared by heating ethyl chloride with alcoholic potassium hydroxide.
![]()
Reactions of Ethylene:
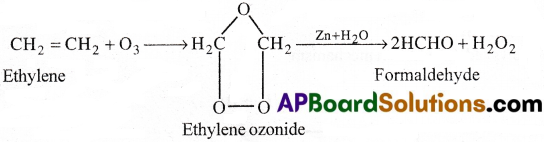
a) Action with Ozone (Ozonolysis):
Ethylene reacts with ozone to form unstable ozonide. This undergoes hydrolysis in presence of Zinc and gives Formaldehyde.
b) Action with hypohaloos acid:
Ethylene reacts with hypochlorous acid and gives Ethylene chlorohydrin.
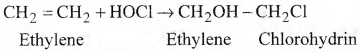
c) Action with cold dil. alk. KMnO4:
Ethylene reacts with cold dil .alk.KMnO4 (Baeyer’s reagent) at 273K and gives Ethylene glycol.

In this reaction, Baeyer’s reagent loses its prime colour. This test is used to detect carbon- carbon double bond or triple bond and is known as Baeyer’s test.
d) Action with O2 (Polymerisation):
Ethylene when heated with 02 at high pressure gives Polythene. [AP 17]

Question 8.
How does ethylene react with the following reagents? Give the chemical equations and names of the products formed in the reactions. [TS 16]
a) Hydrogen.halide
b) Hydrogen
c) Bromine
d) Water
e) Oxygen in presence of Ag at 200°C
f) H2SO4
Answer:
a) Ethylene undergoes addition with hydrogen halides forming ethyl halides.
![]()
b) Ethylene reacts with hydrogen in presence of Pt, Pd or Ni catalyst to form ethane.
![]()
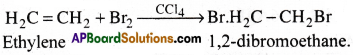
c) Ethylene reacts with bromine in presence of CCl4 to form 1, 2- dibromoethane.
d) When ethylene gas is passed through dil.H2SO4, it undergoes addition with water forming ethyl alcohol.
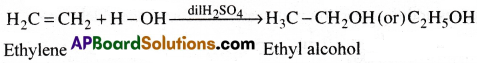
e) Ethylene on heating in oxygen in presence of Ag at 200°C give ethylene oxide.

f) Ethylene reacts with H2SO4 to form ethylene hydrogen sulphate
CH2 = CH2 + H2SO4 → CH3.CH2.HSO4
![]()
Question 9.
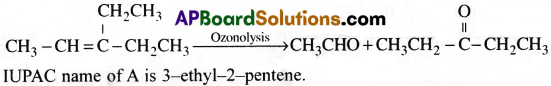
An alkene ‘A’ on ozonolysis gives a mixture of ethanal and pcntan3-onc. Write the reaction, structure of the products and alkene-A. Give the IUPAO name of alkene A.
Answer:
Question 10.
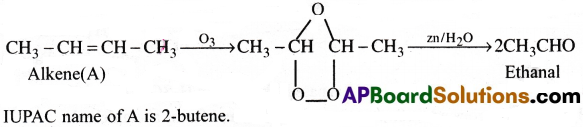
An alkene ‘A’ contains three C-C, eight C-H bonds and once C=C bond. ‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44. Write IUPAC name of ‘A’.
Answer:
The aldehyde with molar mass 44 is CH3CHO(ethanal).
Question 11.
Give two methods of preparation of acetylene. How does it react with water and ozone? [AP 20][TS 18,20]
Answer:
Preparation of Acetylene (C2H2):
1) Dehydro halogenation :
Acetylene is prepared by heating dibromoethane with alcoholic KOH. This undergoes dehydrohalogenation and gives acetylene.
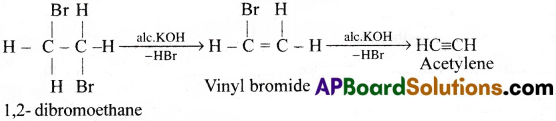
2) From iodoform:
Acetylene is prepared by heating Iodoform with silver powder.
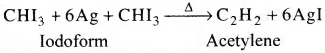
Properties of Acetylene:
1) Action with water:
Acetylene gas when passed through dil.H2SO4 below 60°C in the presence of H2SO4, undergoes addition reaction with water and forms acetaldehyde. [ AP 17]
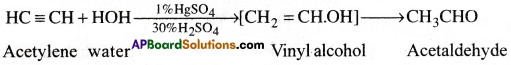
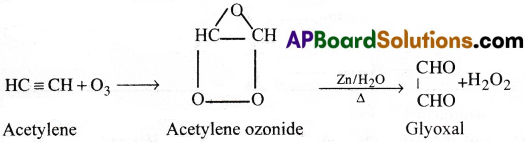
2) Action with ozone:
Acetylene reacts with ozone to form acetylene ozonide. This on hydrolysis in the presence of Zn forms glyoxal.
Question 12.
How does acetylene react with the following reagents? Give the corresponding equations and name the products formed in the reactions. [TS 16,18]
a) Acetic acid
b) Water
c) Hydrogen
d) Halogens
e) Hydrogen halide
f) Ammonial AgNO3 and Cu2Cl2.
Answer:
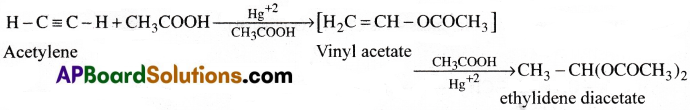
a) Action ith CH3COOH:
Acetylene reacts with acetic acid in presence of Hg+2 ions as catalyst, forming at first vinyl acetate and finally ethylidene diacetate.
b) Action with water:
Acetylene gas when passed through dil.H2SO4 below 60°C in the presence of H2SO4, undergoes addition reaction with water and forms acetaldehyde.
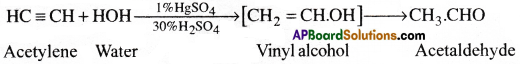
c) Action with Hydrogen:
Acetylene reacts with H2 in presence of Ni or Pt catalyst and undergoes addition reaction forming ethylene and ethane.

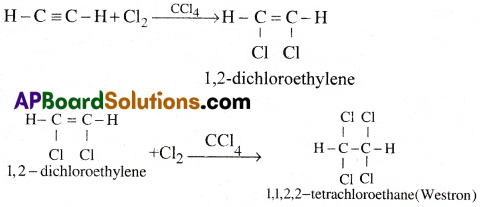
d) Action with Halogens:
Acetylene reacts with halogens in presence of CCl4 and undergoes addition reaction giving finally 1, 1,2, 2 -tetrachloroethane.
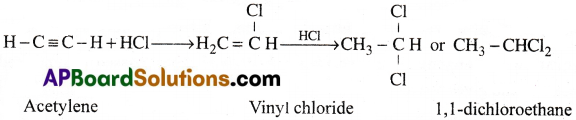
e) Action with Hydrogen halides:
Acetylene undergoes addition with hydrogen halides and gives finally 1,1 -dichloroethane. [TS 20]
f) Action with ammoniacal AgNO3 solution:
when acetylene gas is passed through ammoniacal AgNO3 solution, a white ppt. of silver acetylide is formed.

g) Action with ammonical Cu2Cl2 solution:
When acetylene gas is passed through ammoniacal Cu2Cl2 solution, a red ppt.of cuprous acetylide is formed.

Question 13.
Describe any two methods of preparation of benzene with corresponding equations. Benzene does not behave like an alkenes, why? How do we get methyl benzene from benzene? [AP 19]
Answer:
Preparation of Benzene:
Laboratory Method:
Sodium benzoate on distillation with soda lime gives benzene.

A mixture of NaOEl and CaO is called soda lime.
2) Polymersiation of acetylene:
Acetylene when passed through red hot Cu, polymerises and gives benzene.
![]()
Reason for not behaving as an alkenc:
Benzene has a number of resonance structures.
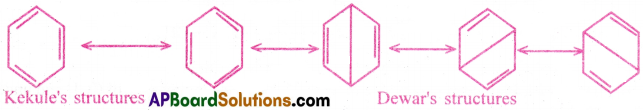
Because of resonance, benzene has more stability . The resonance energy of benzene is also very high. It also indicates more stability to the benzene molecule. Hence, stability of a molecule is due to saturation. Hence, from these two points it is evident that benzene exhibits more the properties of a saturated compound (alkane) rather than the properties of an unsaturated compound (alkene).
Methyl benzene from benzene :
In the presence of anhydrous AlCl3, benzene reacts with methyl chloride and forms methyl benzene (Toluene).

Question 14.
How do we get benzene from acetylene? Give the corresponding equation. Explain the halogenation, alkylation, acylation, nitration and sulphonation of benzene. [AP 15][TS 19][AP 18]
Answer:
Preparation of benzene from acetylene:
Acetylene when passed through red hot Cu, polymerises and gives benzene.
![]()
1) Halogenation :
Benzene reacts with chlorine in the presence of Anhy. AlCl3 and forms. [AP 18][IPE’ 14]

2) Friedel Craft’s alkylation :
Benzene reacts with methyl chloride in the presence of anhy. AlCl3 and forms methyl benzene. [AP 18,22][IPE 13,14]
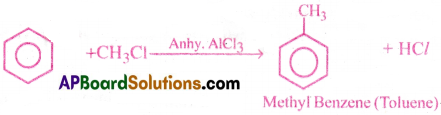
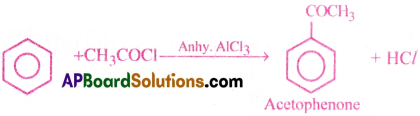
3) Friedel – Craft’s acylation:
Benzene reacts with acetyl chloride in the presence of anhy. AlCl3 and forms acetophenone.
4) Nitration :
Benzene reacts with Nitric acid in the presence of cone. H2SO4 at 60°C and forms nitro benzene. [May’13][AP 17,22]

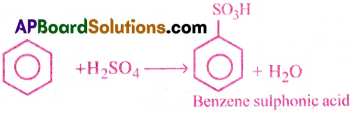
5) Sulphonation:
Benzene reacts with filming sulphuric acid and gives benzene sulphonic acid.
Question 15.
Explain the differences between structural isomers and stereo isomers.
Answer:
| Structural Isomers | Stereo Isomers |
| 1) The compounds having same moiecuiar formula but differ in their structural arrangements of atoms or group of atoms | 1) The compounds having same moiecuiar formula but differ in their spatial arrangement of atoms or group of atoms. |
| 2) Spatial arrangement is not considered. | 2) Structure of the compound is not changed. |
| 3) Strucutral isomers possess different physical properties. Ex: With increase in number of branches in alkanes with same molecular fomrula, boiling points are decreased. |
3) Stereo isomers may or may not differ in their physical properties. Ex: a)Diastereomers and Cis-trans isomers differ in their boiling points and melting points. b) Enantiomers have same physical properties like melting & boiling points. |
| 4) Chain isomers, positional isomers, functional isomers, metamers, keto-enol tautomers and ring isomers are called structural isomers. | 4) Configurational and conformational isomers are called stereoisomers. |
![]()
Question 16.
What is the difference between conformation and configuration in open chain molecules.
Answer:
Stereo isomers are two types known as configurational and conformational isomers.
Conformation isomers:
These are the stereo isomers that are easily converted into one another by the rotation around ‘C-C’ (Sigma ) bonds. These are in dynamic equilibrium with one another. This type of isomerism is seen in alkanes. Ex: n-butane.
Configuration Isomers:
They cannot be interconverted into one another with out making and breaking of new bonds. Configurational stereo isomers possess certain types of rigidity in the molecules which are discrete, stable.
Question 17.
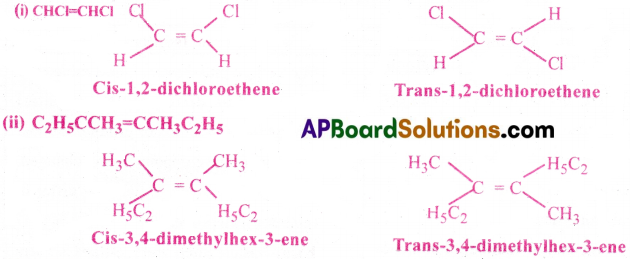
What do you understand about geometrical isomerism? Explain the geometrical isomers of 2-butene. Draw the cis and trans isomers of (i) CHCl=CHCl (ii) C2H5CCH3=CCH3C2H5 [AP 22]
Answer:
Geometrical isomerism arises due to restricted rotation of atoms or groups along C=C bond.
Ex: Geometrical isomers of 2-butene are as follows.

The isomer in which same groups or atoms are on the same side of the C=C is called cis-isomer while the isomer having same groups or atoms on the opposite sides of the C=C is called trans¬isomer. In cis-2-Butene the two methyl groups are on one side while the two hydrogen atoms are on the other side. In trans-2-butene two methyl groups and two hydrogen atoms are present opposite to the double bond. This isomerism is due to restricted rotation around C=C bond.
Question 18.
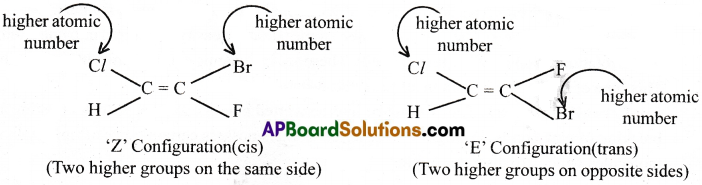
Explain the method of writing E – Z configurations for geometrical isomers taking CHCl = CFBr as an example.
Answer:
E – Z configurations for geometrical isomers – Steps :
i) Arrange the atoms/groups attached to each doubly bonded carbon in the order of their atomic numbers.
ii) Choose the atom/group of higher priority on each doubly bonded carbon. If the atoms/groups of higher priority on each carbon are on the same side of the molecule, the letter ‘Z’ is used to denote the configuration of such isomer. When the atoms/groups of higher priority on each carbon are on the opposite sides of the molecule, the letter ‘E’ is prefixed before the name to indicate configuration.
Ex : CHCl = CFBr
Among H and Cl, 17Cl gets more priority than 1H.
Among F and Br, 35Br gets more priority than 9F.
![]()
Question 19.
If an alkene contains on carbons at double bond Cl.Br-CH2-CH2-OH and -CH(CH3)2. Write the E and Z configurations of it.
Answer:

Question 20.
Write a note on : (a) Distillation (b) Fractional distillation
(c) Distillation under reduced pressure (d) Steam distillation
Answer:
a) Distillation :
- This process is useful for the purification of liquids contaminated with nonvolatile impurities.
- The impure liquid is boiled in a distillation flask and the vapours are condensed and collected in a receiver.
- This method can also be used to separate liquids if only their boiling points differ by above 40°C.
b) Fractional distillation :
- It is used to separate a liquid mixture which contains the components that have boiling point difference less than 40°C.
- Let a mixture of A and B is taken in a distillation flask which is fitted with a fractionating column at its mouth.
- The upper end of the column is connected to the water condenser.
- When the mixture is heated, the vapours of both A and B pass through the fractionating column.
- While moving through column, the vapours of liquid which has high boiling point (sayA) condenses and come back to the flask.
- The vapours of other liquid (say B) which has low boiling point still in vapour state and move out of column as pure vapours of B to the condenser.
- Vapours of B condense and get collected in the receiver.
c) Distillation under reduced pressure :
- This method is useful to purity liquids that have very high boiling points and those which decompose at or below their boiling points.
- If the external pressure is reduced the liquid boils at lower temperature than its normal boiling point without decomposition.
- At reduced pressure the boiling point of a liquid is also reduced, hence its decomposition is avoided
Ex: Glycerol and H2O2 are purified by this method.
d) Steam distillation :
- This method is used to purify the liquids which are insoluble in water, possess high vapour pressure and steam volatile.
- In this method steam is passed into the hot liquid mixture taken in the distillation flask.
- The liquid with low boiling point comes out along the steam by leaving, behind the liquid which has comparatively high boiling point.
- They are passed through the condenser and condensed and finally collected in the receiver.
- The water layer and the organic liquid layer are separated using a separating funnel. Aniline can be purified by this method.
Question 21.
Write a brief note on Chromatography.
Answer:
Chromatography is developed as a method of separating components of a mixture based on adsorption, generally between the stationary phase and a mobile phase.
Chromatography involves the three steps given below :
a) Adsorption and retention of a mixture of substances on the stationary phase and separation of adsorbed substances by the mobile phase to different distances on the stationary phase.
b) Recovery of the substances separated by a continuous flow of the mobile phase (known as elution)
c) Qualitative and quantative analysis of the eluted substances.
Classification :
Two general chromatography techniques are
i) adsorption chromatography
ii) partition chromatography.
Question 22.
Explain the following:
(a) Column chromatography
(b) Thin layer chromatography
(c) Partition chromatography
Answer:
a) Column chromatography :
- In the column chromatography ,the components of a mixture are separated by a column of adsorbent packed in a glass tube.
- The column is fitted with a stopcock at its lower end.
- The mixture to be adsorbed on the adsorbent is placed at the top of the starionary phase.
- A suitable eluant, either a single solvent or a mixture of solvents is allowed to flow down the column slowly.
- Depending on the degree to which the compounds are adsorbed the components are separated.
- The most readily absorbed substances are retained near the top and other come down accordingly to various distances.
b) Thin layer chromatography (TLC):
- This also involves adsorption differences. Here the adsorbent, say silica gel or alumina is coated over a glass plate of suitable size in thin layer.
- The plate is called TLC plate or chromoplate.
- The solution of the mixture to be separated is applied as a small spot at about 2 cm from the bottom of the plate.
- The plate is then kept in a closed jar containing the eluant,
- As the eluant rises up the plate, the components of the mixture move up along with the eluant to various distances depending on their degree of adsorption.
- The relative adsorption of a component of the mixture is expressed in terms of its Retardation Factor (Rf) value.
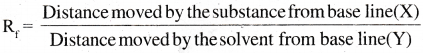
Partition chromatography :
- This principle is similar to thin layer chromatography, except that a strip of paper acts as adsorbent.
- It is based on continuous differential separation of components of a mixture between the stationary phase and the mobile phase.
- In paper chromatography, for example, a special paper called chromatography paper contains water trapped in it which acts as the stationary phase.
- The chromatography paper spotted with the solution of the mixture at the base is suspended in a suitable solvent or a mixture of solvents.
- The solvent rises up the paper by capillary action and moves over the spot.
- The paper selectively retains different components as per their differing partition in mobile and stationaiy phase.
- The paper strip so developed is known as chromatogram.
- The spots of the separated coloured compounds are detected and for colourless compounds other methods like spraying suitable reagent are used.
Question 23.
Explain the estimation of nitrogen of an organic compound by
a) Dumas method
b) KjeldahPs method
Answer:
a) Estimation of nitrogen by Duma’s method:
In this method a known weight of organic compound is heated strongly with coarse cupric oxide. Then carbon and hydrogen get oxidised to CO2 and H2O vapour respectively. Nitrogen is converted to N2 gas. Some nitrogen converted into its oxides, gets reduced by copper gauze to nitrogen. The liberated gases are passed over a solution of KOH. Then CO2 gets absorbed. Nitrogen is collected over KOH solution and its volume is found out.
Calculations:
Suppose ‘a’ g of nitrogen compound gives V1 ml of N2 gas at room temperature T K. If atmospheric pressure is P and aqueous tension at TK is P0, then the pressure of N2 gas at TK is P1 = (P – P0).

b) Estimation of N2 by Kjeldahl’s method:
In this method the organic compound is heated with conc. H2SO4 in presence of a small amount of CuSO4. Then N2 is quantitatively converted into ammonium sulphate. The contents of the flask are transferred into another flask and heated with excess of NaOH solution to liberate ammonia gas. The gas so liberated is passed and absorbed in a known vol. of known cone. H2SO4(excess). Now the excess of acid remained after the neutralisation by NH3 is titrated against a standard solution of alkali. From this, the amount of H2SO4 used to neutralise NH3 is calculated. From this the mass of ammonia formed is calculated and from that the % of N2 gas is calculated.
Organic compound + H2SO4 → (NH4)2SO4
(NH4)2SO4 + 2NaOH → Na2SO4 + 2H2O + 2NH3
2NH3 + H2SO4 → (NH4)2SO4
Calculation:
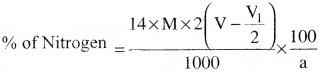
a = Weight of organic compound = a g
M = Molarity of H2SO4 solution
V = Volume of H2SO4 solution
V1 = Volume of H2SO4 solution consumed by NaOH.
Question 24.
Explain Inductive effect with a suitable example.
Answer:
Inductive effect :
The permanent displacement of sigma (a) bonded electrons towards the more electronegative atoms (or) group is known as Inductive effect.
Illustration :
Consider the molecue CH3-CH2-CH2-Cl. There is a ‘σ’ covalent bond between carbon atom and chlorine atom. The electron pair between them is not equally shared. The more electronegative chlorine atom tends to attract the sharped pair, more towards itself. Due to this, the electron density tends to be greater, nearer chlorine atom than carbon atom.
This is generally represented as ![]() . But, carbon atom bonded to chlorine atom is itself attached to other carbon atoms. Therefore, the effect can be transmitted further.
. But, carbon atom bonded to chlorine atom is itself attached to other carbon atoms. Therefore, the effect can be transmitted further.
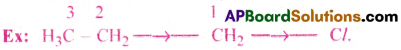
Typcs of Inductive effect:
-I effect:
Electron withdrawing groups are said to have negative inductive effect. An order of -I effect
-NO2 > CHO > CO > COOH > F > Cl > Br > I > OH > OR > NH2 > C6H5 > H.
+ I effect :
Electron donating groups are said to have positive inductive effect.
An order of+1 effect: -C(CH3)3 >-CH(CH3)2 > -CH2CH3 > -CH3
![]()
Question 25.
Write a on Mesomeric effect.
Answer:
Mesomeric effect :
The permanent transfer of ‘π’ electrons from multiple bonds to adjacent atom (or) bond (or) transfer of lone pair of electrons from an atom to adjacent bond is known as mesomeric effect.
Salient features of the mesomeric effect :
- Mesomeric effect is a permanent effect and it operates in the ground state of the molecule.
- Lone pairs and π electrons are involved in this effect and this operate through eonjugative mechanism of electron displacement.
- This effect influences the physical properties, reaction rates etc.
Types of mesomeric effect :
+M effect:
Groups which tend to increase the electron density of the rest of the molecule are said to have +M effect. Such groups tend to posses lone pairs of electrons.
Ex: H2N-C=C- Here – NH2 group has + M effect.
-M effect:
Groups that decrease the electron density of the rest of the molecule are said to have -M effect. Unsaturated groups having polar character have -M effect. Ex: -C=C-C=0 Here C=0 group decreases the electron density of the remaining molecule.Hence it has -M effect.
Question 26.
Describe Resonance effect with one example.
Answer:
Resonance effect:
This is the polarity produced in a molecule by the interactions of two π bonds or between a π bond and a lone pair of electrons present on adjacent atoms. This effect i s tran sm itted through the chain.
If the transfer of electron is away from the atom or substituent group attached to the conjugated system, then the molecule gets some of its positions high electron density as in aniline and it is given +R.
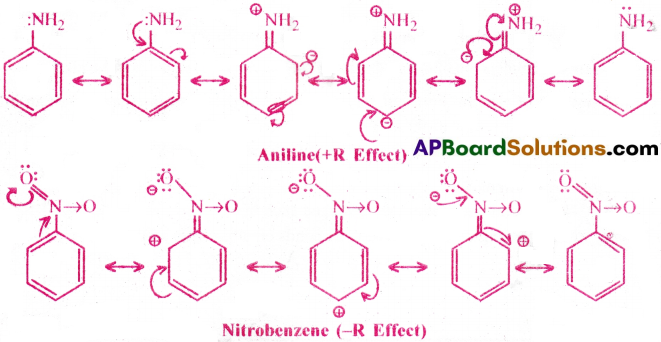
If the shift of electrons are towards the atom or substituent group it is (-R) as in nitrobenzene and it is given -R
Groups showing (+R) effect : X, -OH, -OR, -NH2, -NHR, – NR2, -NHCOR
Groups showing (-R) effect : – COOH, CHO, > C = O, -CN, – NO2.
Question 27.
Explain how many types of organic reactions are possible.
Answer:
Organic reactions are mainly classified into four types known as
i) Addition reactions
ii) Substitution reaction
iii) Elimination reactions
iv) Molecular rearrangements.
i) Addition reactions :
In these reactions the reagent and the substrate combine together to give a single product.

Depending on the reagent added in the slow rate determining step, addition reactions are agian classified as a) Electrophilic addition reactions b) Nucleophilic addition reactions c) Free radical addition reactions.
ii) Substitution reactions :
In these reactions an atom or a group of the substrate species is replaced by another atom or group. These are again classified as a) electrophilic substitution b) Nucleophilic substitution and c) Free radical substitution reactions on the basis of the reagent involved in the rate determining step.
Ex: OH–(aq) + R – X → HO – R + X–(aq)
iii) Elimination reactions :
In these reactions two or more atoms or groups of an organic substrate are removed to form multiple
CH3CH2Br + KOH(alc) → CH2 = CH2 + KBr + H2O
iv) Molecular rearrangements :
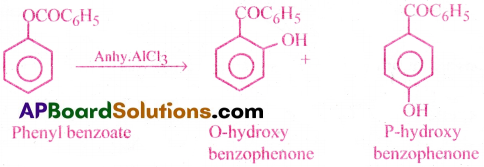
Flere one organic species (generally less stable) rearranges to other species (generally more stable). For example, Fries rearrangement.
Question 28.
Write the possible conformations of ethane and epxlain which is more stable. [Mar’ 09]
Answer:
Conformational isomers are obtained due to rotation about C-C single bond in alkanes.
Conformations of ethane:
Free rotation about C-C single bond in ethane gives an infinite number of conformers. Out of these, Eclipsed conformation and Staggered conformation are most significant. All the remaining conformers of ethane are called Skew conformations.
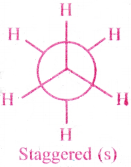
1) Staggered confirmation :
The C-H bond on one carbon are arranged such that they bisects the angle between two C-H bonds on adjascent carbon.
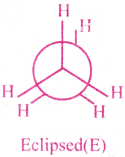
2) Eclipsed confirmation :
Each O H bond on one carbon are aligned with C-H bonds on adjacent (C-H bond) carbon.
Stagged conformer is most stable than the eclipsed. Because in Eclipsed conformer of ethane hydrogen atoms are very close to each other. So, repulsions are more and stability is less. The difference in the energy content of Staggered and Eclipsed Conformations is 12.5kJmol-1.
Question 29.
Explain aromatic electrophilic substitution reactions of benzene.
Answer:
Electrophilic substitution reaction of benzene proceeds in three steps.
Step I :
Generation of electrophile: The reagent undergoes heterolysis to form electrophile.
E – A → E⊕ + AᎾ
Step 2:
The electrophile (E+) takes two electrons of the six-electron π system, to form a σ bond with a carbon atom of the benzene ring. Then that carbon atom becomes sp³ hybridised. As a result carbocation is formed which is stabilised by resonance.
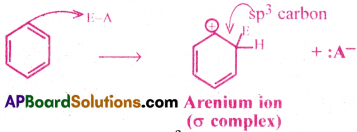
Step 3:
A proton is removed from the carbon atom of the σ complex that bears the E (electrophile). Then that carbon atom changes to sp² state again. Then the benzene derivative with fully delocalised six π electrons is formed.
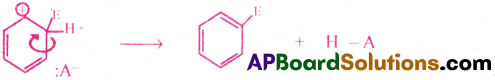
(The proton H+ combines with the anion obtained from the molecule E – A → H+ + A– → HA)
Question 30.
Explain electrophilic addition reactions of ethylene with mechanism.
Answer:
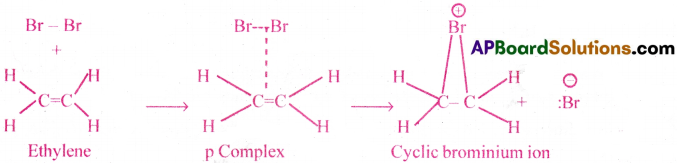
1) Addition of halogens:
Ethylene combines with halogens at room temperature in an inert solvent medium to give a vicinal dihalide.
Ex: Bromination.
![]()
Mechanism: It involves 2 steps.
Step(1):
The π-electrons of the double bond attack the halogen molecule.Then a loose π complex (cyclic bromonium ion), with a 3- membered ring is formed.
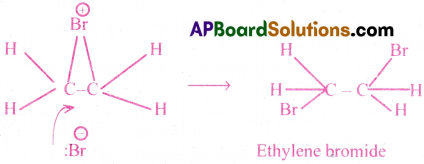
Step(2) :
The negative bromide ion attacks the cyclie bromonium ion from the backside.
This is called ‘trans’ addition of halogens.
2) Addition of hydrogen halide:
Ethylene reacts with hydrogen halide (HX) to give ethyl halide.
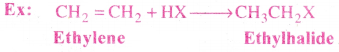
Mechanism:
This mechanism is quite similar to that of the halogen addition, involving 2 steps
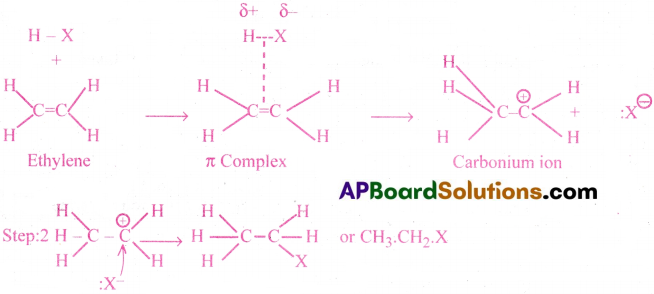
![]()
Question 31.
With the help of mechanism explain free radical halogenations of alkanes.
Answer:
Chlorination of ethane takes place in three steps known as (1) Chain Initiation (2) Chain Propagation and (3) Chain Termination.
i) Chain Initiation:
in this step Cl2 molecule absorbs energy and splits into Cl free radicals. (C-C bonds and C-H bonds of ethane being relatively stronger, they do not break at this stage.)

ii) Chain Propagation:
Chlorine free radicals react with ethane molecule.
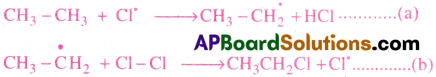
(a) and (b) repeat several times making the reaction a chain reaction and these steps are called propagation steps. In these steps the main products are formed.
In addition to (a) and (b) other reactions to replace other hydrogen atoms of ethane also take place.
iii) Chain Termination:
When free radicals directly combine the chain reaction stops down.
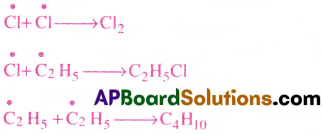
Question 32.
Explain Markownikoff’s rule and Kharash effect(Anti-markownikoff).
Answer:
Addition of Hydrogen halides:
Addition of HX to a double bond generally follows Markownikoff s rule.
Statement of Markownikoff s rule:
The rule states that addition of hydrogen halides to unsymmetrical alkenes takes place in such a manner that the positive part of the reagnet attach itself to that carbon atom which has more number of hydrogen atoms.
Explanation:
It follows electrophilic addition mechanism.
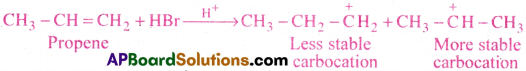
Since 2° carbocation is more stable than l° carbocation, the major product formed is 2-Bromo propane.
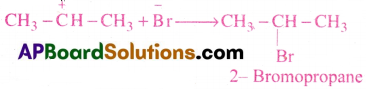
Note: Markownikoff s rule is only applicable for unsymmetrical alkenes.
Anti Markownikoffs rule or peroxide effect or Kharasch effect:
In the presence of peroxide (R-O-O-R) the addition of HBr to unsymmetrical alkene like propene takes place in such a way that the negative part of the reagent attach itself to that carbon atom which has more number of hydrogen atoms.
Stability of free radicals follows as 30>2°>1°. Therefore, 1-bromopropane is major prodcut
Uses of Ethylene : Ethylene is usded
- In the manufacture of ethylalcohol, polythene etc.
- In the preparation of ethylene glycol an antifreeze.
- In the preparation of mustard gas.
Question 33.
How would you convert the following compounds into benzene? a) Chlorobenzene b) Toluene c) p-nitro toluene
Answer:
a) Reduction of chlorobenzene with Ni-Al alloy andNaOH gives benzene.
C6H5Cl + 2[H] → C6H6 + HCl
b) Toluene is first oxidised to benzonic acid with dil.HNO3 and alkaline or acidic KMnO4. Then decarboxylation of benzoic acid gives benzene.
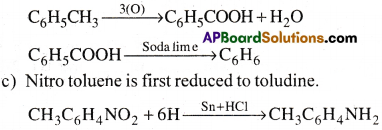
This on dizotization forms diazonium salt which on treating with KCN/Cu followed by hydrolysis gives p-methyl benzoic acid. The methyl group is also oxidised to acid group with dil. HNO3. This on decarboxylation gives benzene.
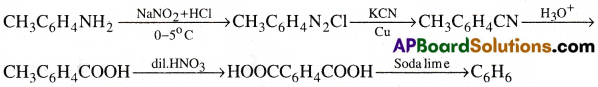
Question 34.
Why is Wurtz reaction not preferred for the preparation of alkanes containing odd number of carbon atoms? Illustrate with one example?
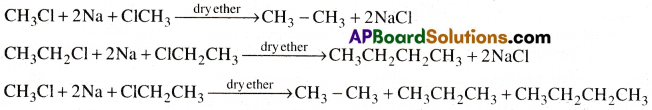
Answer:
In Wurtz reaction when alkyl halide is treated with sodium metal, an alkane having double the number carbon atoms present in alkyl halide will be formed. So we always get an alkane with even number of carbon atoms. If an alkyl halide with even number of carbon atoms and another alkyl halide with odd number of carbon atoms are used in Wurtz reaction a mixture of hydrocarbons are produced. So Wurtz reaction is not preferable for the preparation of alkanes with odd number of carbon atoms.
This can be illustrated with the following examples.
Question 35.
Write the equations involved in the detection of Nitrogen, Halogens and sulphur in organic compounds.
Answer:
Lassaigne’s Test:
In Lassaigane’s test, the organic compound is mixed with sodium metal and fused strongly. The fused mass is extracted with water by plunging the red hot ignition tube in distilled water and the contents are boiled for 5 minutes and filtered. The filtrate is called Sodium fusion extract or Lassaigne’s extract.
If nitrogen is present in the organic compound, it reacts with sodium and carbon forming sodium cyanide.
Na + C + N → NaCN
If sulphur is present in the organic compound, it reacts with sodium forming sodium
2Na + S → Na2S
If halogens are present in the organic compound, it reacts with sodium fonning sodium halides.
Na + X → NaX [X= Cl, Br, I]
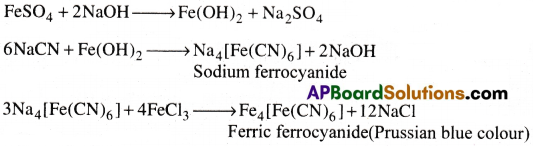
Test for Nitrogen:
A little of the Lassaigne’s extract is made alkaline with NaOH solution and then freshly prepared FeSO4 solution is added. To it 2-3 drops of FeCl3 solution is added.
A prussian blue colouration is observed.
Test for Halogens:
A little of the Lassaigne’s extract is acidified with nitric acid and treated with AgNO3 solution.
If white ppt. soluble in NH4OH is formed, the halide is Cl–. Hence, chlorine is present.
If pale yellow ppt. partially soluble in NH4OH is formed, the halide is Br–. Hence, bromine is present.
If yellow ppt. insoluble in NH4OH is formed, the halide is I–. Hence, iodine is present.
Test for Sulphur:
To a little of the Lassaigne’s extract freshly prepared sodium nitroprusside solution is added. Deep purple colouration is observed.
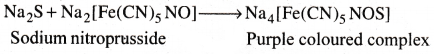
![]()
Question 36.
Explain how Carbon and Hydrogen are quantitatively determined in an organic compound.
Answer:
Liebig’s method for the quantitative estimation of carbon and hydrogen:
A known weight of the organic compound is taken and completely burnt in excess of air and copper(II) oxide. Then carbon oxidises to CO2 and hydrogen oxidises to H2O. The CO– and H2O so obtained are passed through already weighed U tubes containing anhydrous CaCl2 and caustic potash respectively. The increased weights of these two tubes give the weights of H2O and CO2 formed.
Suppose that ‘a’ g of organic compound on combustion gives ‘b’ g of water vapourand ‘c’ g of CO2.
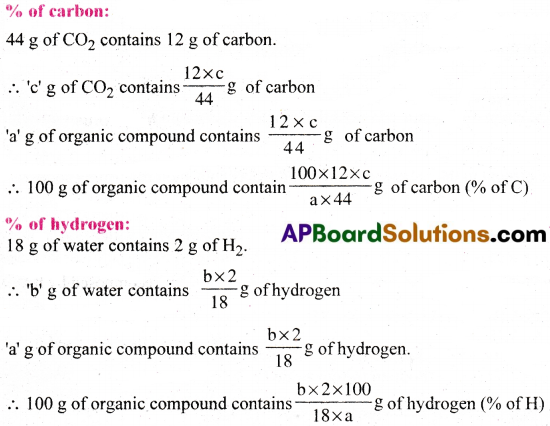
Question 37.
Describe Dumas and Kjeldahl’s method for the estimation of Nitrogen.
Answer:
a) Estimation of nitrogen by Duma’s method:
In this method a known weight of organic compound is heated strongly with coarse cupric oxide. Then carbon and hydrogen get oxidised to CO2 and H2O vapour respectively. Nitrogen is converted to N2 gas. Some nitrogen converted into its oxides, gets reduced by copper gauze to nitrogen. The liberated gases are passed over a solution of KOH. Then CO2 gets absorbed. Nitrogen is collected over KOH solution and its volume is found out.
Calculations:
Suppose ‘a’ g of nitrogen compound gives V1 ml of N2 gas at room temperature T K. If atmospheric pressure is P and aqueous tension at TK is P0, then the pressure of N2 gas at TK is P1 = (P – P0).

b) Estimation of N2 by Kjeldahl’s method:
In this method the organic compound is heated with conc. H2SO4 in presence of a small amount of CuSO4. Then N2 is quantitatively converted into ammonium sulphate. The contents of the flask are transferred into another flask and heated with excess of NaOH solution to liberate ammonia gas. The gas so liberated is passed and absorbed in a known vol. of known cone. H2SO4(excess). Now the excess of acid remained after the neutralisation by NH3 is titrated against a standard solution of alkali. From this, the amount of H2SO4 used to neutralise NH3 is calculated. From this the mass of ammonia formed is calculated and from that the % of N2 gas is calculated.
Organic compound + H2SO4 → (NH4)2SO4
(NH4)2SO4 + 2NaOH → Na2SO4 + 2H2O + 2NH3
2NH3 + H2SO4 → (NH4)2SO4
Calculation:
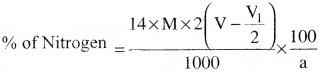
a = Weight of organic compound = a g
M = Molarity of H2SO4 solution
V = Volume of H2SO4 solution
V1 = Volume of H2SO4 solution consumed by NaOH.
Question 38.
How do you determine Sulphur, Phosphorous and Oxygen are determined quantitatively in an organic compound?
Answer:
Estimation of Sulphur:
A known mass of the organic compound is heated with sodium peroxide in a Carius tube. Then ‘S’ is oxidised to H2SO4. The acid is precipitated as BaSO4 by adding excess of BaCl2 aq. solution. The ppt. is filtered, washed, dried and weighed.
Mass of organic compound = a g
Mass of barium sulphate = b g
Molecular weight of BaSO4 = 233
l mole of BaSO4 or 233 g of BaSO4 contains 32 g of sulphur.

Estimation of phosphorous:
A known mass of organic compound is heated with fuming nitric acid in a Carius tube. Then phosphorus is oxidised to phosphoric acid. The acid is precipitated as ammonium phosphomolybdate by adding ammonia and ammonium molybdate solutions.
Calculation:
Mass of organic compound = a g.
Mass of ammonium phosphomolybdate = b g.
Molecular weight of ammonium phosphomolybdate = 1877.
1877 g of ammonium phosphomolybdate contains 31 g of phosphorus

Estimation of Oxygen:
A known weight of organic compound is decomposed by heating in a stream of nitrogen gas. The mixture of gaseous products containing oxygen is passed over red hot coke to convert all oxygen in those oxides to CO. Then the mixture is passed through hot iodine pentoxide to convert to CO into CO2 and iodine liberates.
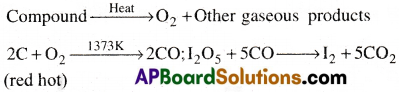
Calculation:
Mass of organic compound = a g
Mass of carbon dioxide = b g
44 g of CO2 contains 16 g of oxygen.

![]()
Question 39.
Explain Carius method for the determination of Halogens quantitatively in an organic compound.
Answer:
Halogens can be estimated by Carius method.
In this method a known weight of the organic compound is heated with fuming nitric acid in presence of AgNO3 in a hard glass tube. Carbon and hydrogen are oxidised to CO2 & H2O. Halogens are converted into silver halides. The silver halide is filtered off, washed, dried and weighed.
Calculation:
Mass of the organic compound = ‘a’ g.
Mass of the silver halide formed (AgX) = ‘b’g.
I mole of AgX contains l mole of X
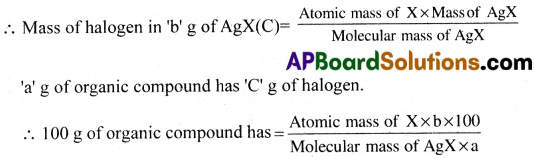
(Molecular Masses: AgCl = 143.5(108 + 35.5); AgBr = 188 (108 + 80); Agl = 235 (108 + 127)
Question 40.
What is Carcinogenicity? Explain with two examples?
Answer:
Benzene and several polynuclear hydrocarbons are toxic and are said to be carcinogenic (Cancer producing).
Most of these are formed due to incomplete combustion of organic substances like tobacco, coal, petroleum etc.
They undergo various chemical changes in human body and finally damage DNA to cause cancer.
Multiple Choice Questions
Question 1.
Which of the following is the correct SUPAC name?
1) 3-Ethyl-4,4-dimethylheptane
2) 4,4-Dimethyl-3-ethylheptane
3) 5-Ethyl-4,4-dimethylheptane
4) 4,4-Bis(methyl)-3-ethylheptane
Answer:
2) 4,4-Dimethyl-3-ethylheptane
Question 2.
The IUPAC name for
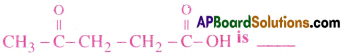
1) 1-hydroxypentane-1,4-dione
2) 1,4-dioxopentanol
3) 1-carboxybutan-3-one
4) 4-oxopentanoic acid
Answer:
4) 4-oxopentanoic acid
Question 3.
The IUPAC name for is
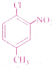
1) 1 -Chloro-2-nitro-4-methylbenzene
2) 1 -Chloro-4-methyl-2-nitrobenzene
3) 2-Chloro-1 -nitro-5-methy lbenzene
4) m-Nitro-p-chlorotoluene
Answer:
2) 1 -Chloro-4-methyl-2-nitrobenzene
![]()
Question 4.
The number of sigma (σ) and pi(π) bonds in pent-2-en-4-yne is
1) 13 σ bonds and no π bond
2) 10 σ bonds and no 3π bonds
3) 8 σ bonds and no 5π bonds
4) 11 σ bonds and no 2π bonds
Answer:
2) 10 σ bonds and no 3π bonds
Question 5.
In which of the following, functional group isomerism is not possible?
1) Alcohols
2) Aldehydes
3) Alkyl halides
4) Cyanides
Answer:
3) Alkyl halides
Question 6.
Dihedral angle of least stable conformer of ethane is
1) 0°
2) 120°
3) 180°
4) 60°
Answer:
1) 0°
Question 7.
Arrange the following in decreasing order of their boiling points.
(A) 77-butane
(B) 2-methylbutane
(C) 77-pentane
(D) 2,2-dimethylpropane
1) A > B > C > D
2) B > C > D > A
3) D > C > B > A
4) C > B > D > A
Answer:
4) C > B > D > A
Question 8.
The compound which shows metamerism is
1) C4H10O
2) C5H12
3) C3H8O
4) C3H6O
Answer:
1) C4H10O
![]()
Question 9.
During hearing of a court case, the judge suspected that some changes in the documents had been carried out. He asked the forensic department to check the ink used at two different places. According to you which technique can give the best results?
1) column chromatography
2) solvent extraction
3) distillation
4) thin layer chromatography
Answer:
4) thin layer chromatography
Question 10.
The principle involved in paper chromatography is
1) Adsorption
2) Partition
3) Solubility
4) Volatility
Answer:
2) Partition
Question 11.
Paper chromatography is an example of
1) adsorption of chromatography
2) partition of chromatography
3) thin layer of chromatography
4) column chromatography
Answer:
2) partition of chromatography
Question 12.
The increasing order of reduction of alkyl halides with zinc and dilute HCl is
1) R-Cl < R-I < R-Br
2) R-Cl < R-Br < R-I
3) R-I < R-Br < R-Cl
4) R-Br < R-I < R-Cl
Answer:
2) R-Cl < R-Br < R-I
Question 13.
Arrange the following hydrogen halides in order of their decreasing reactivity with propene.
1) HCl > HBr > HI
2) HBr > HI > HCl
3) HI > HBr > HCl
4) HCl > HI > HBr
Answer:
3) HI > HBr > HCl
![]()
Question 14.
Arrange the halogens F2, Cl2, Br2, I2, in order of their increasing reactivity with alkanes.
1) I2 < Br2 < Cl2 < F2
2) Br2 < Cl2 < F2 < I2
3) F2 < Cl2 < Br2 < I2
4) Br2 < I2 < Cl2 < F2
Answer:
1) I2 < Br2 < Cl2 < F2
Question 15.
Arrange the following carbanions in order of their decreasing stability.
(A) H3C – C ≡ C–
(B) H – C ≡ C–
(C) H3C-CH–2
1) A > B > C
2) B > A > C
3) C > B > A
4) C > A > B
Answer:
2) B > A > C
Question 16.
A tertiary butyl carbocation is more stable than a secondary butyl carbocation because of which of the following?
1) -I effect of -CH3 groups
2) +R effect of -CH3 groups
3) -R effect of -CH3 groups
4) Hyperconjugation
Answer:
4) Hyperconjugation
Question 17.
Which of the following is correct with respect to -I effect of the substituents? (R=alkyl)
1) -NH2 < -OR < -F
2) – NR2 < OR < F
3) – NH2 > -OR > -F
4) – NR2 > -OR > -F
Answer:
1) -NH2 < -OR < -F
Question 18.
In which of the following compounds the carbon marked with asterisk is expected to have greatest positive charge?
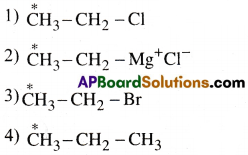
Answer:
1
Question 19.
Which of the following alkane cannot be made in good yield by Wurtz reaction?
1) n-Hexane
2) 2,3-Dimethylbutane
3) n-Heptane
4) n-Butane
Answer:
3) n-Heptane
![]()
Question 20.
![]()
Consider the above reaction and identify the missing reagent/chemical
1) DIBAL-H
2) B2H6
3) Red Phoshporous
4) CaO
Answer:
4) CaO