Students get through AP Inter 1st Year Chemistry Important Questions 12th Lesson Environmental Chemistry which are most likely to be asked in the exam.
AP Inter 1st Year Chemistry Important Questions 12th Lesson Environmental Chemistry
Very Short Answer Questions
Question 1.
Define the terms atmosphere, biosphere.
Answer:
Atmosphere:
The protective blanket of gases present around the earth is called atmosphere.
Biosphere:
The part of the earth in which all living organisms exist is called biosphere.
Question 2.
Explain the terms Lithosphere, Hydrosphere.
Answer:
Hydrosphere:
- All the natural water resources like oceans, seas, rivers, lakes, glaciers, polar ice caps and ground water constitute the hydrosphere.
- Water occupies 3/4th of the earth’s surface. Out of this 97% is present in the form of sea water and the remaining 3% in the form of ice in polar ice caps.
- Small percentage of water is available for drinking, agriculture and other human purposes.
Lithosphere:
- The outer mantle of the solid earth consisting of minerals and the soil is called Lithosphere.
- The inner layers of earth contain minerals, the deeper inner layers contain natural gas and oil.
Question 3.
Define the term Soil Pollution.
Answer:
Any factor which reduce the quality, texture and mineral content of the soil and disturbs the biological balance of living organisms in it is called soil pollution.
Question 4.
What is Chemical Oxygen Demand (COD)? [AP 18; AP,TS 15,16,17,19]
Answer:
Chemical Oxygen Demand (COD):
The amount of oxygen required to oxidise organic substances present in polluted water is chemical oxygen demand (COD).
Question 5.
What is Biochemical Oxygen Demand (BOD)? [TS 18,19,19] [AP 15, 16,18,20]
Answer:
Biochemical Oxygen Demand (BOD):
The amount of oxygen used by suitable microorganisms present in water during five days at 20°C is called B.O.D. For pure water BOD is about 1 ppm.
![]()
Question 6.
What are Troposphere and Stratosphere?
Answer:
Troposphere:
The lowest region of atmosphere in which the human beings along with other organisms live is called troposphere.
Stratosphere:
Above the troposphere, extends from 10 to 50 km above sea level is called stratosphere.
Question 7.
Name the major particulate pollutants present in Troposphere. [AP 19]
Answer:
Particulate pollutants present in troposphere are dust, mist, smoke and smog.
Question 8.
List out four Gaseous Pollutants present in the polluted air.
Answer:
Oxides of Sulphur, Nitrogen and Carbon, Ozone, Hydrocarbons etc., are gaseous pollutants present in polluted air.
Question 9.
Green house effect is caused by …..and ……..gases. [AP,TS 20][IPE’ 14][TS 18]
Answer:
Green house effect is caused by gases such as CO2, CH4, O3, CFCs (Chloro Fluoro Carbons) and water vapour in the atmosphere.
Question 10.
Which oxides cause acid rain? What is its pH value? [IPE 13][TS 19]
Answer:
Oxides of Nitrogen, Sulphur and Carbon dissolved in rain water causes acid rain.
The pH value of acid rain is 5.6.
Question 11.
Name two adverse effects caused by acid rains. [AP, TS 15,17,18][AP 16]
Answer:
- Acid rains reduce the life of buildings and historical monuments.
- Acid rains decrease the fertility of soil by reducing the pH values of the soil.
- Acid rains decrease quality of drinking water.
- Acid rains decrease the productivity of fish in water.
![]()
Question 12.
What are smoke and mist?
Answer:
Smoke:
A mixture of CO2, Water vapour with very small soot particles produced by burning and combustion of organic matter are called smoke.
Mist :
The particles produced by spray liquids, formed by condensation of vapours in air is called mist.
Question 13.
What is classical smog? and What is its Chemical Character (Oxidizing (or) reducing)?
Answer:
Classical smog occurs in cool humid climate. It is a mixture of smoke, fog and sulphur dioxide. Chemically it is a reducing mixture and so it is also called as reducing smog.
Question 14.
Name the common components of Photochemical smog. [AP 16,19]
Answer:
Common components of photochemical smog are ozone, nitric oxide, formaldehyde, acrolein and PAN (peroxy acetyl nitrate). It is also called as oxidising smog.
Question 15.
What is PAN? What effect is caused by it? [AP 19]
Answer:
PAN means Peroxy acetyl nitrate is called
PAN. It causes photochemical smog.

Question 16.
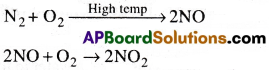
How is Ozone formed in the Stratosphere?
Answer:
The formation of O3 in stratosphere takes place in two steps.
In the first step the Ultraviolet radiation coming from sun split the dioxygen into two oxygen atoms. In second step, the oxygen atoms react with the molecular oxygen to form ozone.

![]()
Question 17.
Give the Chemical equations involved in (he Ozone depletion by CF2 Cl2.
Answer:
CFC’s undergo decomposition in the presence of sunlight as given below.
Reactions:

Chain reaction continues in which ozone layer is depleted.
Question 18.
What is Ozone hole? Where was it first observed?
Answer:
The depletion of ozone layer is commonly known as ozone hole. It was first observed in Antarctica region of south pole.
Question 19.
What is the value of dissolved Oxygen in pure cooled water?
Answer:
The value of dissolved oxygen in pure cooled water is 4-6 mg/lit (or) 4-6ppm.
Question 20.
Give the possible BOD values of clean water and the polluted water.
Answer:
For pure water BOD is 1 ppm.
BOD value of clean water is less than 5 ppm.
BOD value of polluted water is 17ppm or more.
Municipal Sewage -l 00-4000 ppm.
Question 21.
Name three industrial Chemicals that pollute water.
Answer:
Poly chlorinated biphenyls, detergents and fertilizers.
![]()
Question 22.
What agrochemicals arc responsible for water pollution? [AP 19]
Answer:
Fertilizers, insecticides, herbicides, fungicides etc are responsible for water pollution.
Short Answer Questions
Question 1.
What are different segments of the earth’s environment?
Answer:
Environment is divided into four segments.
They are 1) Atmosphere
2) Hydrosphere
3) Lithosphere and
4) Biosphere.
1) Atmosphere:
The blanket of gases that surrounds the earth is called Atmosphere. It contains large proportions N2 and O2 and in small proportions CO2., water vapour etc. It absorbs the harmful radiations coming from the sun and plays an important role in maintaining the heat balance on earth.
2) Hydrosphere:
All the natural water resources together constitute the Hydrosphere. It includes oceans, seas, rivers, lakes, streams, reservoirs, polar ice caps and ground water etc.
3/4th of the earth’s surface is occupied by water. Out of this 97% is present in the form of sea water and the remaining 3% is in the form of ice in polar ice caps and only very small percentage of water is available for drinking, agriculture and other human purposes.
3) Lithosphere:
1/4th of the total earth surface is in the form of land Inner layers of earth contain minerals. Deeper inner layers of earth contain natural gas and oil. All these things including hills and mountains come under this segment.
4) Biosphere:
All living organisms, trees, plants, animals, and human beings constitute Biosphere. This biosphere is interrelated to other segments of environment. Biosphere is dependent on atmosphere and hydrosphere. Polluted atmosphere stops the plants growth and can create health problems among animals and human beings.
Question 2.
Define the terms Sink, COD, BOD and TLV. [TS 15,16,18]
Answer:
Sink :
The medium which interacts with pollutants and reduces its effect is called sink.
COD (Chemical Oxygen Demand):
The amount of oxygen required to oxidise organic substances present in polluted water is called chemical oxygen demand (COD).
BOD(Biochemicai Oxygen Demand):
The amount of oxygen used by the suitable micro-organisms present in water during five days at 20°C is called B.O.D.
TLV(Threshold Limit Value):
The permissible level of a toxic pollutant without any adverse effect on a healthy industrial worker, working for 8 hours per day, in a polluted atmosphere is called TLV.
Question 3.
Name the gaseous pollutants present in the air and explain their formation.
Answer:
Substances which mix with air and affect the human beings, animals, plants and global temperature are called air pollutants.
Examples:
- Oxides of carbon: CO and CO2
- Oxides of Nitrogen: N2O and NO
- Oxides of Sulphur: SO2
- Ozone
- CFCs: Chlorofluorocarbons
- Methane and Butane
- Smog
- Metals: Lead, Mercury.
Air pollution :
In cities, 80% of air pollution is due to exhausts of automobiles. At peak times in cities the level of CO will be upto 50-100ppm. If it is inhaled, it causes adverse effects.
During the combustion of fossil fuels NO, N2O, NO2 etc. are also released. When the level of these oxides is greater than 10 ppm, the plants cannot perform photosynthesis.
SO2 is released into the atmosphere during the burning of sulphur and roasting of sulphide ores. SO2 causes respiratory diseases in human beings. It bleaches the green colour of the leaf apexes in plants to yellow colour and thus prevent photosynthesis process.
Chlorofluoro carbons when percolate into stratosphere cause depletion of ozone layer. Harmful pesticides and biocides mix up with the air at their manufacturing units and pollute the air. They cause major health hazards.
Question 4.
What is Green house effect? and how is it caused? [TS 15][AP 18]
Answer:
The progressive warming up of the earth’s surface due to blanketing effect of CO2 and water vapour in the atmosphere is called global warming (or) green house effect.
Reason :
If the concentrations of gases such as CO2, NO, N2O, CH4, O3 etc increase in atmosphere, the gases act like the glass panels of a green house (or) the window glass of a closed car. They allow the sunrays to the earth, but prevent the longer wavelength (Infrared) radiations coming from the earth. As a result, more energy is radiated back to the Earth by the gases due to their increased concentration and their blanketing effect. Hence the temperature of the earth increases.
Effects :
- The ice caps of polar region melt. This results in the increase of the level of the sea water upto 90cm. Because of this, low lying countries will get submerged.
- The rate of evaporation of water from the seas, river, ponds will increase. This leads to cyclones and hurricanes.
- Agriculture sector will be affected badly.
Preventions:
- The number of sinks (Plants) should be increased to absorb CO2.
- The growth of blue-green algae in sea water should be prevented.
Question 5.
Explain with chemical equation involved the formation of acid rain.
Answer:
Acid rain :
Acid rain is the rainwater containing sulphuric acid and nitric acid which are formed from the oxides of sulphur and nitrogen present in the air as pollutants and has a pH of 4 – 5.
Formation of acid rain:
Oxides of nitrogen combine with oxygen and ozone to form higher oxides of nitrogen. These oxides dissolve in water to form nitric acid.
NO + O3 → NO2 + O2
NO2 + O3 → NO3 + O7
NO2 + NO2 → N7O5
N2O5 + H2O → 2HNO3
Sulphur dioxide reacts with oxygen and water to form sulphuric acid.
SO7 + ½O2 → SO3
SO3 + H2O → H2SO4
These acids obtained in the atmosphere dissolve in rain water and come down to earth as rain. It is called acid rain.
![]()
Question 6.
Explain in detail the adverse effects caused by the acid rain.
Answer:
- Acid rain is harmful for agriculture. It washes away the nutrients required for the growth of plants. So crops cannot grow in the areas where the acid rain falls.
- Aquatic animals and plants cannot live in acid water. So they die in the lakes, ponds, rivers where acid rain falls.
- The metal pipes which carry water corrodes in the acid water where the acid rain falls.
- Acid rain damages buildings and other structures made with stone or metal.
Ex: Tajmahal in India has been affected by acid rain.
Question 7.
How is photochemical smog formed? What arc its ill effects.
Answer:
Photochemical smog is produced from the action of sunlight on nitrogen oxides and hydrocarbons present in the exhaust gases of the automobiles and factories. The NO is oxidised by the atmospheric oxygen to NO2.
NO2 then undergoes photochemical decomposition in the presence of sunlight.
![]()
The free oxygen atom so formed reacts with atmospheric oxygen to form ozone.
O2(g) + O(g) → O3(g)
Ozone then reacts rapidly with NO(g) to form NO2(g)
NO(g) + O3(g) → NO2(g) + O2(g)
Ozone is a toxic gas and both NO2 and O2 are strong oxidising agents and can react with the unburnt hydrocarbons in the polluted air to produce chemicals such as formaldehyde, acrolein and peroxyacetyl nitrate (PAN). The common components of photochemical smog are ozone, nitric oxide, acrolein, formaldehyde and PAN. It is also called as oxidising smog.
Effects:
- Photochemical smog causes eye irritation.
- It decreases visibility and thus effects the air and road traffic.
- It leads to cracking of rubber and extensive damage to plant life.
Question 8.
How is ozone layer depleted in the atmosphere and what are the harmful effects caused by ozone layer depletion. [TS 15,16]
Answer:
The process of destroying the existence of ozone molecules in the stratosphere is called depletion of ozone layer.
Depletion of ozone layer is due to release of certain chemical substances like CFC’s, NO, Cl2 and gases evolved from Volcanoes.
CFC’s absorb U.V radiation and decompose to liberate chlorine free radicals which catalyse the decomposition of ozone, causing depletion of ozone layer.
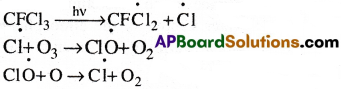
Effects of ozone holes:
The ‘ozone layer’ prevents the U V rays coming from the Sun. When holes are formed in ozone layer, then U.V. rays will pass through these holes and reach the earth. These U.V. rays cause (1) Skin cancer (2) Cataract of eyes (3) Decrease moisture content of the soil (4) Decrease in the efficiency of photosynthesis in plants
Question 9.
List out the industrial wastes that cause water pollution and what are the international standards fixed for drinking water.
Answer:
Process Wastes:
These are form inorganic process wastes and organic process wastes. Inorganic process wastes are present in the effluents from chemical industries, electroplating industries, metallurgical and petroleum industries etc. These are mainly toxic but do not generally produce biological problems. Organic process wastes are from food processing industries, diaries, distilleries, paper, textile mills etc. It is very difficult to dispose organic process wastes.
Chemical Wastes :
Industries that manufacture acids, bases, detergents, explosives, dyes, insecticides, fungicides, fertilisers, silicones, plastics, resins etc., which are generally used as raw materials for further manufacturing processes, contain chemical wastes. These wastes are produced during sedimentation, flocculation, washing, filtering, evaporation, distillation, electrolysis, absorption, crystallisation, burning, centrifusing etc. Chemical wastes require biological oxidation treatment methods like thickening filters, activated sludge method. The international standards fixed for drinking water.
Fluoride concentration – 1 ppm
Lead – 50 ppb
Sulphate -<500 ppm
Nitrate – 50 ppm
![]()
Question 10.
Explain in detail the strategies adopted in Green chemistry to avoid environmental pollution.
Answer:
The ways of using the knowledge and the principles of chemistry and other sciences to develop methods to reduce the pollution of the environment as far as possible are known as green chemistry.
The over exploitation of the soil using fertilisers and pesticides polluted the soil, water and air. But farming irrigation etc cannot be stopped. Then, methods to reduce the environmental pollution must be developed.
Generally in a reaction some by-products are formed. In many processes these by-products become the pollutants. Green chemistry works for not producing wasteful by-products in these processes.
Normal process:
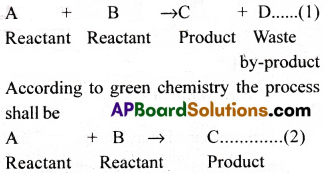
As the reaction (2) gives no by-product it is called an environmental-friendly reaction. Even if a by-product is formed, it must be made a useful product instead of polluting the environment. Green chemistry suggests that instead of using conventional fuels and energy systems, non-non-conventional fuels and non-conventional systems must be put into practice. Because of this, pollution would be reduced.
Green chemistry is a cost effective approach that involves minimum chemical usage, minimum energy consumption and minimum wastef pollutant) generation.
Ex: In the dry cleaning of clothes, tetrachloroethane was used earlier. This compound contaminates the ground water. Therefore, this compound is replaced by liquefied CO2 along with a suitable detergent. This would not pollute ground water much. Nowadays, H2O2 is used for bleaching clothes in laundries. This gives better results and decreases the consumption of water.
Long Answer Questions
Question 1.
What is environmental pollution? How many types of pollution are encountered?
Answer:
Due to increase in the population and industrialisation, the natural resources have diminished. To prepare many natural things artificially, many industries were started. For improving the yields many technologies were introduced. Along with this development, many waste products were released into environment. Thus environment got polluted. This is known as environmental pollution.
Some reasons for environmental pollution are:
- Increase in population and decrease in natural resources.
- Industrialisation
- Urbanisation
- Deforestation
The different types of pollutions are:
- Air pollution
- Water pollution.
- Soil pollution
- Sound pollution
- Thermal pollution
- Radiological pollution
Question 2.
Explain the following in detail.
a) Global warming b) Ozone depletion c) Acid Rain d) Eutrophication
Answer:
a) Global Warming :
Gases like CO2, CFCs, O3, NO and water vapour absorb I.R radiations coming to the earth and reflect them back to the earth’s surface. Due to this, the surface of the earth gets heated. This is called greenhouse effect or global warming. The gases which are responsible for this effect are called greenhouse gases.
Effect of global warming: [AP 17]
- For a 1°C rise in temperature, the ice caps of polar regions melt and level of the sea water increases. Thereby many coastal countries get submerged.
- Due to global warming, the rate of evaporation of water from the seas, rivers, ponds will increase. This leads to unwarranted rains, cyclones and hurricanes.
- Due to global wanning, short of water supply for agriculture occurs. Global warming can be prevented by growing trees, forests, stopping production of CFCs etc.
b) Ozone depletion: Ozone layer is depleted by the pollutant gases such as ehlorofluorocarbons, NO2 and HOCl.
The Chlorofluoro carbons break down in the presence of sunlight and deplete the ozone layer as follows.
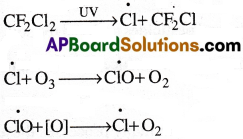
The chloride radicals are continuously regenerated and cause the breakdown of ozone. One CFC molecule destroys one lakh O3 molecules.
In winter season the Cl\(\dot{\mathrm{O}}\) reacts with NO2 forming chlorine nitrate. This hydrolyses giving hypochlorous acid and HCl. In summer season, these breakup providing C\(\dot{\mathrm{ḷ}}\) radicals which can deplete the ozone layer.
c) Acid Rain:
Oxides of nitrogen and sulphur released from automobiles and industries enter into atmosphere and dissolves in water to form the acids HNO3 and H2SO4. These acids dissolve in rain water and come down to earth as acid rain.
2SO2 + 2H2O + O2 → 2H2SO4
4NO2 + 2H2O + O2 → 4HNO3
- Acid rain is harmful for agriculture. It washes away the nutrients required for the growth of plants. So crops cannot grow in the areas where the acid rain falls.
- Aquatic animals and plants cannot live in acid water. So they die in the lakes, ponds, rivers where acid rain falls.
- The metal pipes which carry water corrodes in the acid water where the acid rain falls.
- Acid rain damages buildings and other structures made with stone or metal.
Ex: Tajmahal in India has been affected by acid rain.
d) Eutrophication :
When organic waste from industry and agriculture are released into ponds or lakes, then the lakes become over bounded with over nutrients and large amounts of phosphates. These support the growth of algae. Thus the lake becomes marshy, filled with sediments and ultimately becomes dry. This process is known as eutrophication.
![]()
Question 3.
Green Chemistry is to avoid environmental pollution. Explain.
Answer:
The ways of using the knowledge and the principles of chemistry and other sciences to develop methods to reduce the pollution of the environment as far as possible are known as green chemistry.
The over exploitation of the soil using fertilisers and pesticides polluted the soil, water and air. But farming irrigation etc cannot be stopped. Then, methods to reduce the environmental pollution must be developed.
Generally in a reaction some by-products are formed. In many processes these by-products become the pollutants. Green chemistry works for not producing wasteful by-products in these processes.
Normal process:

As the reaction (2) gives no by-product it is called an environmental-friendly reaction. Even if a by-product is formed, it must be made a useful product instead of polluting the environment. Green chemistry suggests that instead of using conventional fuels and energy systems, non-conventional fuels and non-conventional systems must be put into practice. Because of this, pollution would be reduced.
Green chemistry is a cost effective approach that involves minimum chemical usage, minimum energy consumption and minimum waste( pollutant) generation.
Ex: In the dry cleaning of clothes, tetrachloroethane was used earlier. This compound contaminates the ground water. Therefore, this compound is replaced by liquefied CO2 along with a suitable detergent. This would not pollute ground water much. Nowadays, H2O2 is used for bleaching clothes in laundries. This gives better results and decreases the consumption of water.
Multiple Choice Questions
Question 1.
Which of the following gases is not a green house gas?
1) CO
2) O3
3) CH4
4) H2O vapour
Answer:
1) CO
Question 2.
Excessive release of CO2 into the atmosphere results in
1) global warming
2) formation of smog
3) polar vortex
4) depletion of ozone.
Answer:
1) global warming
Question 3.
Photochemical smog occurs in warm, dry and sunny climate. One of the following is not amongst the components of photochemical smog, identify it.
1) NO2
2) O3
3) SO2
4) Unsaturated hydrocarbon
Answer:
3) SO2
Question 4.
The higher concentration of which gas in air can cause stiffness of flower buds?
1) SO2
2) CO
3) NO2
4) CO2
Answer:
1) SO2
Question 5.
Which oxide of nitrogen is not a common pollutant introduced into the atmosphere both due to natural and human activity?
1) N2O5
2) NO2
3) N2O
4) NO
Answer:
1) N2O5
![]()
Question 6.
Air pollution that occurs in sunlight is
1) oxidising smog
2) fog
3) reducing smog
4) acid rain.
Answer:
1) oxidising smog
Question 7.
The layer of atmosphere between 10 km and 50 km above the sea level is called
1) thermosphere
2) mesosphere
3) stratosphere
4) troposphere.
Answer:
3) stratosphere
Question 8.
The upper stratosphere consisting of the ozone layer protects us from the sun’s radiation that falls in the wavelength region of
1) 400-550 nm
2) 600-750 nm
3) 200-315 nm
4) 0.8-1.5nm
Answer:
3) 200-315 nm
Question 9.
The gas leaked from a storage tank of the Union Carbide plant in Bhopal gas tragedy was
1) phosgene
2) methylisocyanate
3) methylamine
4) ammonia.
Answer:
2) methylisocyanate
Question 10.
The smog is essentially caused by the presence of
1) O2 and O3
2) O2 and N2
3) oxides of sulphur and nitrogen
4) O3 and N2
Answer:
3) oxides of sulphur and nitrogen
Question 11.
Which of the following is a sink for CO?
1) Microorganisms present in the soil
2) Oceans
3) Plants
4) Haemoglobin
Answer:
1) Microorganisms present in the soil
Question 12.
Which one of the following is not a common component of photochemical smog?
1) Ozone
2) Acrolein
3) Peroxyacetylnitrate
4) Chlorofluorocarbons
Answer:
4) Chlorofluorocarbons
![]()
Question 13.
Which of the following statements is not true about classical smog?
1) Its main components are produced by the action of sunlight on emissions of automobiles and factories.
2) Produced in cold and humid climate.
3) It contains compounds of reducing nature.
4) It contains smoke, fog and sulphur dioxide.
Answer:
1) Its main components are produced by the action of sunlight on emissions of automobiles and factories.
Question 14.
Which of the following is not correct about carbon monoxide?
1) It forms carboxyhaemoglobin
2) It reduces oxygen carrying ability of blood.
3) The carboxyhaemoglobin (haemoglobin bound to CO) is less stable than oxyhaemoglobin
4) It is produced due to incomplete combustion
Answer:
3) The carboxyhaemoglobin (haemoglobin bound to CO) is less stable than oxyhaemoglobin
Question 15.
Which is wrong with respect to our responsibility as a human being to protect our environment?
1) Avoiding the use of floodlighted facilities
2) Setting up compost tin in gardens
3) Using plastic bags
4) Restricting the use of vehicles
Answer:
3) Using plastic bags
Question 16.
BOD stands for
1) Biochemical Oxidation Demand
2) Biological Oxygen Demand
3) Biochemical Oxygen Demand
4) Bacterial Oxidation Demand.
Answer:
3) Biochemical Oxygen Demand
Question 17.
Biochemical Oxygen Demand, (BOD) is a measure of organic material present in water. BOD value less than 5 ppm indicates a water sample to be
1) rich in dissolved oxygen.
2) poor in dissolved oxygen.
3) highly polluted.
4) not suitable for aquatic life.
Answer:
1) rich in dissolved oxygen.
Question 18.
Water samples with BOD values of 4 ppm and 18 ppm, respectively, are
1) clean and highly polluted
2) highly polluted and highly polluted
3) highly polluted and clean
4) clean and clean.
Answer:
1) clean and highly polluted
![]()
Question 19.
Addition of phosphate fertilizers to water bodies causes
1) enhanced growth of algae
2) increase in amount of dissolved oxygen in water
3) deposition of calcium phosphate
4) increase in fish population.
Answer:
1) enhanced growth of algae
Question 20.
Taj Mahal is being slowly disfigured and discoloured. This is primarily due to
1) acid rain
2) soil pollution
3) water pollution
4) global warming
Answer:
1) acid rain
Question 21.
Identify the wrong statement in the following.
1) Acid rain is mostly because of the oxides of nitrogen and sulphur.
2) Chlorofluorocarbons are responsible for ozone layer depletion.
3) Greenhouse effect is responsible for global warming.
4) Ozone layer does not permit infrared radiation from the sun to reach the earth.
Answer:
4) Ozone layer does not permit infrared radiation from the sun to reach the earth.
Question 22.
Which of the following conditions in drinkipg water causes methemoglobinemia?
1) > 50 ppm of chloride
2) > 50 ppm of nitrate
3) > 50 ppm of lead
4) > 100 ppm of sulphate
Answer:
2) > 50 ppm of nitrate
Question 23.
The maximum prescribed concentration of copper in drinking water is
1) 0.05 ppm
2) 3 ppm
3) 5 ppm
4) 0.5 ppm
Answer:
2) 3 ppm
Question 24.
Among the following the one that is not a green house gas is
1) sulphur dioxide
2) nitrous oxide
3) methane
4) ozone
Answer:
1) sulphur dioxide
![]()
Question 25.
Which one of the following substances used in dry cleaning is a better strategy to control environmental pollution?
1) Sulphur dioxide
2) Carbon dioxide
3) Nitrogen dioxide
4) Tetrachloroethylene
Answer:
2) Carbon dioxide