Students get through AP Inter 1st Year Chemistry Important Questions 1st Lesson Atomic Structure which are most likely to be asked in the exam.
AP Inter 1st Year Chemistry Important Questions 1st Lesson Atomic Structure
Very Short Answer Questions
Question 1.
What is the charge, mass and ‘charge to mass ratio’ of an electron?
Answer:
Charge of an electron = -1.602 × 10-19 C;
Mass of an electron = 9.11 × 10-31 kg
Charge to mass ratio (\(\frac{e}{m}\)) = 1.76 × 10-31 C kg-1
Question 2.
Calculate the charge of one mole of electrons.
Answer:
Charge of one mole of electrons
= Charge of electron × Avogadro number
= 1.6021 × 10-19 × 6.023 × 1023
= 9.6500 × 104 = 96500 C = 1 Faraday
Question 3.
Calculate the mass of one-mole of electrons.
Answer:
Mass of one mole of electrons
= Mass of electron × Avagadro number
= 9.1 × 10-31 × 6.023 × 1023 = 54.8 × 10-8 Kg
= 5.48 × 10-7 Kg
Question 4.
Calculate the mass of one mole of protons.
Answer:
Mass of one mole of proton
= Mass of proton × Avagadro number
= 1.6726 × 10-27 × 6.023 × 1023 = 10.07 × 10-8Kg
= 1.007 × 10-3 Kg
Question 5.
Calculate the mass of one mole of neutrons.
Answer:
Mass of one mole of neutrons
= Mass of neutron × Avagadro number.
= 1.6749 × 10-27 × 6.023 × 1023
= 10.087 × 10-4 Kg = 1.0087 × 10-3 Kg
![]()
Question 6.
How many neutrons and electrons are present in the nuclei of 136C, 168O, 2412Mg, 5626Fe, and 8838Sr.
Answer:
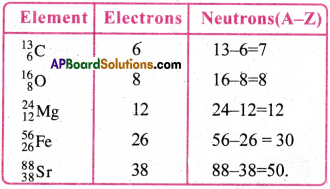
→ Electrons are not present in nucleus.
Question 7.
What is a black body?
Answer:
The ideal body, which emits and absorbs all frequencies of radiation is called a black body.
Ex: A hollow sphere coated inside with platinum black and having a small hole in its walls acts as a near black body.
Question 8.
Which part of electromagnetic spectrum does Balmer series belong?
Answer:
Balmer series (n1 = 2) belongs to visible region of electromagnetic spectrum.
Question 9.
What is an atomic orbital?
Answer:
Atomic orbital is the three dimensional space around the nucleus in an atom, where the probability of finding the electron is maximum (95%).
Question 10.
When an electron is transferred in hydrogen atom from n = 4 orbit to n = 5 orbit to which spectral series does this belong?
Answer:
When an electron is transferred from n = 4 to n = 5, we get the Brackett series in IR region.
Explanation :
By absorbing sufficient energy, the electron in the orbit n = 4 jumps to the orbit n = 5. Now when the electron present in n = 5 returns to the orbit n = 4, it releases energy in the form of Spectral lines, which belongs to Brackett series.
![]()
Question 11.
How many ‘ p’ electrons are present in sulphur atom?
Answer:
The atomic number of sulphur is 16.
So its configuration is 1s² 2s² 2p6 3s² 3p4
The total number of ‘p’ electrons = 6 + 4 = 10.
Question 12.
What are the values of principal quantum number (n), azimuthal quantum number (1) for a 3d electron?
Answer:
For 3d-electron, principal quantum number n = 3 and Azimuthal quantum number l = 2
Question 13.
What is the complete symbol for the atom with the given atomic number (Z) and atomic mass (A)? (I) Z = 4, A = 9; (II) Z = 17, A = 35; (III) Z = 92, A = 233
Answer:
(I) 94Be. (II) 3517Cl (III) 23392U
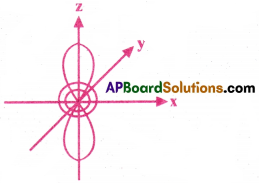
Question 14.
Draw the shape of dZ² orbital.
Answer:
Question 15.
Draw the shape of dx²-y² orbital.
Answer:
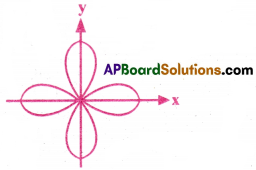
Question 16.
What is the frequency of radiation of wavelength 600nm?
Answer:
Given that λ = 600nm,
We know c= 3,0 × 108ms-1
Formula: c = vλ
⇒ v = \(\frac{\mathrm{c}}{\lambda}=\frac{3.0 \times 10^8 \mathrm{~ms}^{-1}}{600 \times 10^{-9} \mathrm{~m}}\) = 5 × 1014 s-1
Question 17.
What is Zeeman effect?
Answer:
The splitting of spectral lines in the presence of external magnetic field is called Zeeman effect.
![]()
Question 18.
What is Stark effect?
Answer:
The splitting of spectral lines in the presence of strong electric field is called Stark effect.
Question 19.
To which element does the following electronic configuration correspond?
(I) 1s² 2s² 2p6 3s² 3p¹ (II) 1s² 2s² 2p6 3s² 3p6 (III) 1s² 2s² 2p5 (IV) 1s² 2s² 2p²
Answer:
I) Total no.ofelectrons = 2 + 2 + 6 + 2 + 1 = 13
Hence the element with atomic number 13 is Aluminium.
II) Total no.of electrons = 2 + 2 + 6 + 2 + 6 = 18
Hence the element with atomic number 18 is Argon.
III) Total no.of electrons = 2 + 2 + 5 = 9
Hence the element with atomic number 9 is Fluorine.
IV) Total no.of electrons = 2 + 2 + 2 = 6
Hence the element with atomic number 6 is Carbon.
Question 20.
Electrons are emitted with zero velocity from a metal surface when it is exposed to radiation of wavelength 4000A. What is the threshold frequency (ν0)?
Answer:
Given that Wavelength λ = 4000Å.
=4000 × 10-8cm
We know that c = 3 × 1010 cm s-1
velocity v = 0. Then K.E = 0
From the Photoelectric equation, we have
hν = hν0 + KE ⇒ hν = hν0 + 0
∴ hν = hν0 ⇒ ν = ν0 . We know that v = c/λ
∴ ν = ν0 = \(\frac{\mathrm{c}}{\lambda}=\frac{3 \times 10^{10}}{4000 \times 10^{-8}}\) = 7.5 × 1014 s-1
Question 21.
Explain Pauli’s exclusion principle.
Answer:
Pauli’s exclusion principle :
No two electrons in an atom can have the same set of all four quantum numbers.
They differ atleast in one of their quantum numbers.
![]()
Question 22.
What is Aufbau principle? [AP 19]
Answer:
Auf – bau principle :
‘In the ground state of the atoms, the orbitals are filled with electrons, in their increasing order of energies’.
Question 23.
What is Hund’s rule? [AP 19]
Answer:
Hund’s rule :
‘In degenerate orbitals pairing of electrons takes place only after the filling up of available orbitals with one electron each’.
Question 24.
Explain Heisenberg’s uncertainty principle?
Answer:
Heisenberg’s uncertainty principle :
It is impossible to determine simultaneously the exact position and exact momentum of a moving electron around the nucleus.
Mathematically, ∆x.∆p ≥ h / 4 π
Where ∆x = uncertainty in the position,
∆p = uncertainty in momentum,
h = Planck’s constant.
Question 25.
What is the wavelength of an electron moving with a velocity of 2.0 × 107ms-1?
Answer:
Velocity of electron= 2.0 × 107ms-1.
Mass of electron =9.11 × 10-31 kg
Planck’s constant h = 6.625 × 10-34
![]()
Question 26.
An atomic orbital has n = 2, what are the possible values of 1 and m1?
Answer:
| Value of n | l | ml |
| 2 | 0 | 0 |
| 1 | -1, 0, +1 |
![]()
Question 27.
Which of the following orbitals are possible? 2s, 1p, 3f, 2p.
Answer:
1st energy level has only 1s subshell.
2nd energy level has only 2s, 2p subshells.
3rd energy level has only 3s, 3p, 3d subshells.
Hence the possible orbitals among the given are 2s and 2p only.
Question 28.
The static electric charge on the oil drop is -3.2044 × 10-19C. How many electrons are present on it?
Answer:
Charge of one electron = -1.602 × 10-19 C
Total Charge on the oil drop = -3.2044 × 10-19C

Question 29.
Arrange the following type of radiation in increasing order of frequency: (a) X-rays (b) visible radiation (c) microwave radiation and (d) radiation from radio waves.
Answer:
Increasing order of frequencies:
Radio waves < microwaves < visible radiation < X-rays.
Question 30.
How many electrons in an atom may have n = 4, and ms = +1/2?
Answer:
If n = 4 then number of electrons in 4th orbit is 2n² = 2(4)² = 32.
Now out of 32 e–, only 16e– have ms = +1/2
Question 31.
How many sub -Shells are associated with n = 5?
Answer:
If n = 5, then possible T values are l = 0, 1, 2, 3, 4. i.e. s, p, d, f, g (five) subshells.
Question 32.
Explain the particle nature of electromagnetic radiation.
Answer:
- Particle nature of electromagnetic radiation was explained by Max Planck and each particle of light is named as ‘Quanta’.
- The energy of quanta is given by the equation E = hν.
- The particle nature of electro magnetic radiation is explained by Photoelectric effect.
Question 33.
Explain the significance of Heisenberg’s Uncertainty principle.
Answer:
- This principle is significant only for motion of micro particles and is negligible for that of macroscopic objects.
- This principle rules out existence of definite paths (or) trajectories of electrons.
![]()
Question 34.
What series of lines are observed in hydrogen spectra?
Answer:
The series of lines in hydrogen spectra:
| Name of series | Spectra region |
| 1) Lyman | Ultraviolet |
| 2) Balmer | Visible |
| 3) Paschen | Near Infrared |
| 4) Brackett | Infrared |
| 5) Pfund | Far Infrared |
Short Answer Questions
Question 1.
What is the wavelength of light emitted when the electron in a hydrogen atom undergoes transition from an energy level with n = 5 to an energy level with n = 3?
Answer:
Given that n1 = 3, n2 = 5.
We know that RH = 109677cm-1.
From the Rydberg’s equation, we have
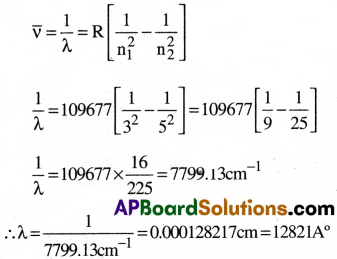
Question 2.
An atom of element contains, 29 electrons and 35 neutrons. Deduce (i) the number of protons and (ii) the electronic configuration of the element?
Answer:
(i) For a neutral atom,
No. of protons = number of electrons = 29
ii) Electronic configuration of element with atomic number Z = 29:
1s²2s²2p63s²3p64s¹3d10
Question 3.
Explain giving reasons, which of the following set of quantum numbers are not possible.
(a) n = 0, I = 0, ml = 0, ms = +1/2
(b) n = 1, I = 0, ml = 0, ms = +1/2
(c) n = 1, I = 1, ml = 0, ms = +1/2
(d) n = 2, I = 1, ml = 0, ms = +1/2
(e) n = 3, I = 3, ml = -3, ms = +1/2
(f) n = 3, I = 1, ml = 0, ms = +1/2
Answer:
Among the given, the following set of quantum numbers are not possible.
(a) n = 0, l = 0, ml = 0, ms = +1/2
Reason :
The values of the principle quantum number ‘n’ are from 1 to n.
Hence n = 0 is not possible.
(c) n = 1, l = 1, ml = 0, ms = +1/2
Reason :
Values of ‘l’ are from 0 to (n – 1) . Thus, if n = 1 then the value of ‘l’ should be 1 – 1 = 0. Hence when n = 1, l = 1 is not possible,
(e) n = 3, l = 3, ml = -3, ms = +1/2
Reason:
If n = 3, possible values of 7′ should be 0,1,2. Hence when n=3,1=3 is not possible.
Question 4.
Show that the circumference of the Bohr orbit for the hydrogen atom is an integral multiple of the Broglie wavelength associated with the electron revolving around the orbit.
Answer:
From Bohr’s model of atom, we have,
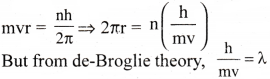
∴ 2πr = nλ, where n is an integer.
Thus the circumference of the orbit is equal to integral multiple of de-Brogile wave length(λ).
![]()
Question 5.
The longest wavelength doublet absorption transition is observed at 589.0 and 589.6 nm. Calculate the frequency of each transition and energy difference between two excited states.
Answer:
(i) Given that λ1 = 589nm = 589 × 10-9m
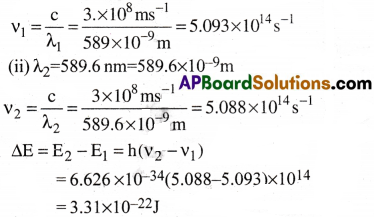
Question 6.
What are the main features of quantum mechanical model of an atom?
Answer:
Important features of quantum mechanical model of atom:
- The energy of electrons in an atom is quantized (it can only have certain specific values)
- The energy levels in an atom are the allowed solutions of Schrodinger wave equation.
- All the information about the orbiting electron in an atom is contained in the orbital wave function ‘Ψ’.
- The exact path of the electron can never be determined accurately. Therefore, we find only the probability of the electron at different points in space, around the nucleus.
- Significance of Ψ² : The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function Ψ². This Ψ² is known as probability density and is always positive.
From the value of Ψ², it is possible to predict the orbital.
Question 7.
What is a nodal plane? How many nodal planes are possible for s, p, d orbitals?
Answer:
Nodal plane is the plane or line passing through the nucleus where the probability of finding an electron is zero.
The number of nodal planes of an orbital is equal to its azimuthal quantum number (l).
Thus No.of nodal planes for ‘s’ orbital = 0 Thus number of nodal planes for any ‘p’ orbital = 1
Thus number of nodal planes for any ‘d’ orbital = 2 (Except dz² = 0)
Question 8.
The Lyman series occurs between 91.2 nm and 121.6 nm, the Balmer series occurs between 364.7nm and 656.5 nm and the Paschen series occurs between 820.6 nm and 1876 nm. Identify the spectral regions to which these wavelengths correspond.
Answer:
| Name of Series | Spectral Region |
| 1) Lyman | Ultra violet |
| 2) Balmer | Visible |
| 3) Paschen | Near infrared |
![]()
Question 9.
How are the quantum numbers n, l, ml for hydrogen atom are obtained?
Answer:
The quantum numbers for hydrogen are obtained as follows:
The atomic number of Hvdrogen = 1
Its electronic configuration is 1s¹.
Hence, the electron revolves in the first orbit. Thus the Principal quantum number is n = 1.
If n = 1 then the Azimuthal quantum number l = n – 1 = 1 – 1 = 0
Its Magnetic quantum number ml = 0
Question 10.
A line in Lyman series of hydrogen atom has a wavelength of 1.03 × 10-7m. What is the initial energy level of the electron?
Answer:
For the line, in Lyman series n1 = 1;
Given Wavelength λ = 1.03 × 10-7m.
Rydbergs constant RH = 1.09 × 10-7m-1.
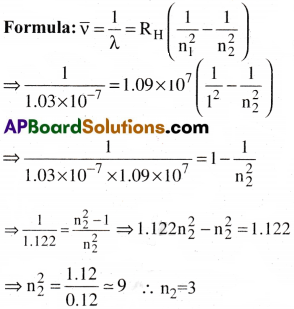
So the electron belongs to 3rd energy level.
Question 11.
If the position of the electron is measured within an accuracy of ±0.002nm. Calculate the uncertainty in the momentum of the electron.
Answer:
Uncertainty in position (∆x) = 0.002nm
Uncertainty in momentum(∆p) = ?
From Heisenberg’s uncertainty principle,

Question 12.
If the velocity of the electron is 1.6 × 106ms-1, calculate the de-Brogile wave length associated with this electron.
Answer:
Velocity of electron v= 1.6 × 106
Mass of electron (m) = 9.1 × 10-31 kg
Planck’s constant (h) = 6.626 × 10-34 Js
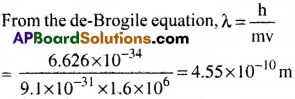
Question 13.
Explain the differences between emission spectra and absorption spectra. |TS 22|
Answer:
| Emission spectrum | Absorption spectrum |
| 1) Emission spectrum is produced due to emission of energy, by an excited substance. | 1) Absorption spectrum is produced due absorption of energy, by a substance. |
| 2) It is produced when electrons jump from higher orbits to lower orbits. | 2) It is produced when electrons transmit from lower orbits to higher orbits. |
| 3) It consists of bright lines or bands on a dark back ground. | 3) It consists of dark lines or bands on a bright back ground. |
| 4) It can be continuous or discontinuous. | 4) It is always discontinuous. |
![]()
Question 14.
The quantum numbers of electrons are given below. Arrange them in order of increasing energies.
a) n = 4, 1 = 2, ml = -2, ms = +1/2
b) n = 3, 1 = 2, ml = -1, ms = -1/2
c) n = 4, 1 = 1, ml = 0, ms = +1/2
d) n = 3, 1 = 1, ml = -1, ms = -1/2
Answer:
Rules:
1) The energy of an electron is calculated by its (n + l) value.( The contributions of m and s are negligible)
2) If two electrons have the same (n + l) value, then the electron enters into lower energy level.
a) If n = 4, l = 2 then n + l = 4 + 2 = 6; 4d
b) If n = 3, l = 2 then n + l = 3 + 2 = 5; 3d
c) If n = 4, l = 1 then n + l = 4 + 1 = 5; 4p
d) If n = 3, l = 1 then n + l = 3 + 1 = 4; 3p
Increasing order of energies:
3p < 3d < 4p < 4d
Question 15.
The work functions for Cesium atom is 1.9eV. Calculate the threshold frequency of the radiation. If the Cesium element is irradiated with a wavelength of 500 nm, calculate the kinetic energy of the ejected photoelectron.
Answer:
Given that Work function(W) = h ν0 = 1.9 eV
= 1.9 × 1.6022 × 10-19 = 3.044 × 10-19J
Wave length(λ) = 500nm = 5 × 10-7m
Now W = h ν0
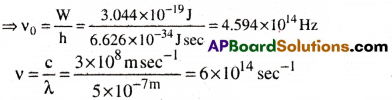
K. E= h(ν – ν0)
= 6.626 × 10-34(6 × 1014 – 4.594 × 1014)
= 6.626 × 10-34 × 1014(6 – 4.594)
= 6.626 × 1020 × 1 .406 = 9.316 × 10-20J
Question 16.
Calculate the wavelength for the emission transition if it starts from the orbit having radius 1.3225 mil and ends at 2II.6 pm. Name the series to which this transition belongs and the region of the spectrum.
Answer:
Calculation of n1 and n2:
Given radius of the orbit in the beginning is r = 1.3225 nm = 1.3225 × 10-9 m
Formula : Radius of the orbit r = 0.529 × n²

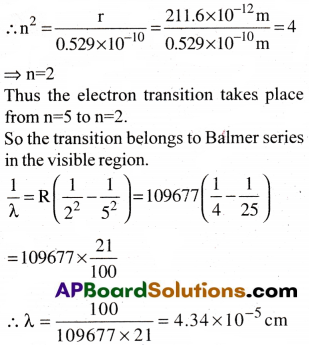
Question 17.
Write the difference between Orbit and Orbital.
Answer:
| Orbit | Orbital |
| 1) Orbit is a well defined circular path of a revolving electron around the nucleus. | 1) Orbital is the 3-dimensional spaced around the nucleus where the probability of finding an electron is maximum. |
| 2) It represents 2- dimensional planar motion of an electron. | 2) It represents three dimensional motion of an electron. |
| 3) The maximum number of electrons in an orbit is 2n², where ‘n’ stands for number of the orbit | 3) The maximum number of electrons in an orbital is 2. |
| 4) Orbits are circular in shape. | 4) Shape of s-orbital is spherical, shape of p-orbital is dumb bell. |
| 5) Orbits are non – directional. They cannot explain shape of molecules. | 5) Orbitals (except s-orbital) are directional. Hence they can account for shapes of molecules. |
| 6) Concept of well-defined orbit is against to Heisenberg’s principle. | 6) Concept of orbital is in accordance with Heisenberg’s principle. |
![]()
Question 18.
Explain photo electric effect on the basis of Einstein’s theory.
Answer:
Einstein’s generalisation of photo electric effect using planck’s quantum theory:
Energy emitted/absorbedby a body, takes place in the form of‘particles of light’ called ‘photons’. A particle in the form of photons, emits energy when it falls from an excited state (E2) to a ground state (E1).
This energy difference ∆E = E2 – E1 is equal to energy of quantum.
Thus, ∆E = E2 – E1 = hν
When a photon strikes the surface of the metal, the energy (hν) of the photon is absorbed by the electron in the metal. A part of this energy is used to ‘set free’ the electron from the ‘attractive forces in the metal’. The remaining part of energy of photons transform in the form of KE of the released electron.
Thus hν = w + KE = hν0 + \(\frac{1}{2}\) meV²
Here,‘w’ is called the work function, ‘w’ denotes the energy required to over come the attractive force on the electron in the metal.
Long Answer Questions
Question 1.
Explain Rutherford’s nuclear model of an atom. What are its drawbacks?
Answer:
Rutherford’s model of atom is based on his ‘α- particle scattering experiment’.
The observations and conclusions of the experiment are given here.
Observation 1 : Most of the α – particles passed through the gold foil were undeflected.
Conclusion : Most of the space in the atom is empty.
Observation 2 : A small fraction of α – particles were deflected by small angles.
Conclusion : The positive charge is not spread through out the atom, as Thomson predicted.
Observation 3 : A ‘very few’ α – particles were bounced back (reflected by 180°).
Conclusion : The positive charge is concentrated in a ‘very small volume’ of the atom.
Rutherford’s nuclear model of atom :
- The positive charge and most of the mass of the atom are concentrated in very small region called Nucleus. (Size of Nucleus = 10-15m)
- The electrons move around the nucleus in circular paths called orbits.
(Thus, Rutherford’s model resembles the solar system, in which the nucleus takes the role of sun and the electrons play the role of revolving planets). - Electrons and nucleus are held together by electro static forces of attraction.Here two equal and opposite forces; centripetal force(towards the nucleus) and centrifugal force(directed away from the orbiting path) balance each other.Hence the electron continues to move in its orbit.
Drawbacks of Rutherford’s model :
- The accelerating electron should emit radiation. Then, by loosing energy, the electron should spiral into the nucleus and hence ultimately the atom should collapse.In fact, this doesn’t happen.
- Else otherwise, if it is assumed that, the electrons won’t accelerate, then they should remain stationary (Just like, in Thomson’s model). In such a case, the dense positive nucleus should pull the negative electrons. But this is also not happening.
- Thus, Rutherford’s model could not explain the stability of atom.
- Rutherford’s model could not explain the electronic structure of atoms. That is, how the electrons are distributed around the nucleus and what are the energies associated with these electrons.
Question 2.
Explain briefly the Planck’s quantum theory.
Answer:
Planck’s quantum theory explains the black body radiation.lt supports particle nature of electro magnetic radiation
Black body :
An ideal body, which emits and absorbs all frequencies of electro magnetic radiation.
Quantum :
The smallest quantity of energy that can be emitted or absorbed discretely in the form of electro magnetic radiation. Quantum is just an energy packet & it has no mass.
Features of Planck’s Quantum theory :
- The emissions of radiation is due to vibrations of charged particles (electrons ) in the body.
- The emissions of radiant energy is not continuous. It happens in the form of small discrete packets of energy called ‘Quanta’.
- The energy of quantum is given by E = hν
Here, h is planck’s constant and ν is the frequency of radiation h = 6.63 × 10-27 erg.s - More over, E = n(hν), n = 1, 2, 3, ……….. Thus, energy is quantised.
That is, the energy emitted or absorbed by a black body, is an integral multiple of ‘quanta’. - ‘Quantums’ will propagate in the form of waves.
- The frequency distribution of the emitted radiations from a black body depends only on its temperature.
![]()
Question 3.
What are the postulates of Bohr’s model of hydrogen atom? Discuss the importance of this model to explain various series of line spectra in hydrogen atom. [AP 15,16,18,19,20,22][TS 16,17,18,20,22]
Answer:
I) Postulates of Bohr’s model of hydrogen atom:
(a) Orbits:
1) The electron in the hydrogen atom can move around the nucleus in a circular path of fixed radius and energy. These paths are called orbits or stationary’ states.
These orbits are arranged concentrically around the nucleus.
2) These orbits are numbered 1, 2, 3, 4 These are known as principal quantum numbers.
As the value of’n’ increases, the size and energy of orbit increases.
(b) Energy of electrons in the orbits :
- The energy of an electron in the orbit does not change with time.
- When sufficient energy is absorbed by the electron, it jumps from the lower orbit to a higher orbit.
- At higher orbits, energy is more but stability’ is less.
- If an electron jumps from higher orbit to lower orbit then it emits energy in the form of photons.
- The photonic transmission causes line spectrum.
- The ‘energy change’ does not take place in a continuous manner.
(c) Frequency of radiation :
If the energy of electron in a lower orbit is E1 and higher orbit is E2 then the change in energy is ∆E = E2 – E1 = h ν. Here, h is Planck’s constant, v is Frequency.
Hence, Frequency ot radiation ν = \(\frac{\Delta \mathrm{E}}{\mathrm{h}}=\frac{\mathrm{E}_2-E_1}{h}\)
(d) Angular momentum of electron :
The angular momentum (mvr) of moving electron is quantised to \(\frac{h}{2 \pi}\). Thus, mvr = n × \(\frac{h}{2 \pi}\)
Here, m = mass of the electron, v = velocity, r = radius of the orbit,
h = Planck’s constant, n = simple integer 1, 2, 3…..
II) Hydrogen spectrum-Bohr’s Explanation:
1) When electric discharge is passed through gaseous hydrogen the electrons in various hydrogen atoms absorb various amounts of energies.
2) Then they enter into higher energy orbits.
3) In higher orbits,the energy is more but stability is less.
4) So, the excited electrons fall back to lower orbits.
5) This happens in one step (or) in multiple steps.
6) Energy is released (in the form of photons) during this process and it appears in the form of spectral lines of Hydrogen spectrum.
7) The transmission of energy of electrons from any higher orbit to
i) n = 1 produces spectral lines in the UV region.This is named as Lyman series.
ii) n = 2 produces spectral lines in the visible region.This is named as Balmer series.
iii) n =3, 4, 5 produces spectral lines in the IR region.These are named as Paschen,Brackett and P-fund series respectively.
8) Formula to find wavenumber(\(\overline{\mathrm{ν}}\)) & wavelength(λ) of spectral lines : \(\overline{\mathrm{ν}}=\frac{1}{\lambda}=\mathrm{R}_{\mathrm{H}}\left[\frac{1}{\mathrm{n}_1^2}-\frac{1}{\mathrm{n}_2^2}\right]\)
9) The hydrogen spectrum is shown here:
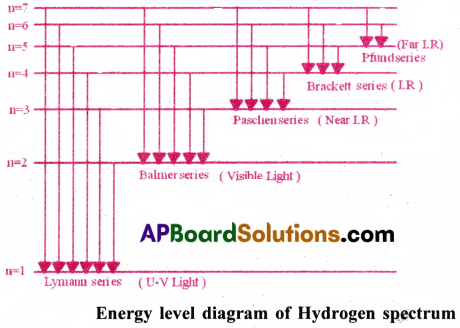
Question 4.
Explain the success of Bohr’s theory for hydrogen atom.
Answer:
Success of Bohr’s theory for hydrogen atom:
1) Bohr’s theory established the quantum numbers :
The stationary states for electron revolving around the nucleus are numbered n = 1, 2, 3 …….. which are known as principal quantum numbers.
2) Bohr’s theory explained the stability of atom :
According to Bohr’s model,an electron revolving in a particular orbit, cannot loose energy. Hence it cannot fall into nucleus. Thus atom remains stable.
3) Bohr’s theory could explain the atomic spectrum of hydrogen :
The different spectral lines in hydrogen spectrum are due to the emission of energy in the form of quanta of radiation. This happens when the excited electron jumps from a higher energy level to a lower energy level.
4) Frequency of radiation :
If the energy of electron in a lower energy level is E1 and higher energy level is E2 then the change in energy is given by ∆E = E2 – E1 = hν.
Here, h is Planck’s constant and ν is Frequency of radiation.
Hence,Frequency of radiation ν = \(\frac{\Delta \mathrm{E}}{\mathrm{h}}=\frac{\mathrm{E}_2-E_1}{h}\) (This is called Bohr’s frequency rule)
The experimentally determined frequencies are in excellent agreement with those calculated by Bohr’s theory.
5) Bohr’s theory is applied to calculate the radius of nth orbit in hydrogen atom using the formula rn = 0.529 × n² Å
From the above equation it is clear that, as ‘n’ increases the value of ‘r’ increases.
When n = 1, the radius of the first stationary state is named as “Bohr Orbit”.
6) Bohr’s theory is applied to calculate the energy of an electron in nth orbit using the
formula En = -RH(\(\frac{1}{n^2}\)), n = 1, 2, 3……… (or) En = –\(\frac{13.6}{n^2}\)eV/atom
Here, RH is called Rydberg constant and its value is 2.178 × 10-18J
The energy level diagram for hydrogen spectrum is drawn using the above formula.
When the electron is free from the influence of nucleus, the energy is taken as ‘zero’ and in this case ‘n’ is assigned ‘∞’.
When the electron is attracted by the nucleus and is present in the orbit ‘n’ then energy is emitted and its energy is lowered. Hence the expression for energy is given the negative sign.
7) Using Bohr’s theory, it is possible to calculate the velocities of electrons moving in the orbits.
8) The angular momentum of electron can be calculated using Bohr’s theory.
9) Bohr’s theory can also be applied to the ions like He+, Li2+, Be3+ which contain only one electron.
Question 5.
What are the consequences that lead to the development of quantum mechanical model of an atom?
Answer:
Classical mechanics prior to quantum mechanical model of atom failed to explain the complete
structure of atom. For example,
- Bohr’s model of atom failed to explain the structure of multi-electronx
xx` atoms. - Classical mechanics failed to explain the motion of microscopic particles like electrons.
- Classic mechanics ignores the dual behaviour of matter.
- The particle nature of electron puts some restriction on finding its position precisely. Heisenberg rectified this limitation in the form of uncertainty principle.
Heisenbergs uncertainty principle :
It is not possible to determine simultaneously the position and momentum of a small moving particle, such as electron with entire certainty. This principle rules out the existence of definite paths or trajectories of electrons and other similar particles.
Thus the precise statements of the position and momentum of electrons are replaced by the. statements of probability of an electron at a given point.
The behaviour of micro particles cannot be explained on the basis of classical mechanics. In order to explain the behaviour of electrons a new branch of science called quantum mechanical model of atom was developed. Quantum mechanics is a theoretical science that takes into account the dual nature of matter.
Louis de-Broglie proposed the dual nature of matter. According to him, microscopic particles such as electrons have both wave & particle-like properties.
Louis de-Broglie derived an equation to calculate the wavelength(λ) of the wave associated with a particle of mass m, moving with velocity v, which is given by
λ = \(\frac{h}{mv}=\frac{h}{p}\)
where ‘h’ is Planck’s constant and p is momentum of the particle.
Question 6.
What are the main features of quantum mechanical model of an atom?
Answer:
Classical mechanics deals with the motion of macroscopic objects (like planets), while
Quantum mechanics deals with the motion of microscopic objects(like electrons).
The microscopic bodies exhibit dual nature. They possess both particle nature and wave nature.
The wave nature of electrons can be depicted from the Schrodinger wave equation.
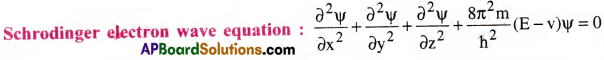
Here, Ψ = amplitude of the electron wave (Ψ is wave function satisfying the wave equation)
m = mass of electron
h = Planck’s constant
E = Total energy ( KE + PE) of the electron
v = Potential energy of the electron
(E-v) = Kinetic energy of the electron
(x, y, z) denote the three dimensional co-ordinates of electron in space.
Main features of quantum mechanical model of atom;
- The energy of electrons in an atom is quantized (it can only have certain specific values)
- The energy levels in an atom are quantised, which are the allowed solutions of Schrodinger wave equation.
- All the information about the orbiting electron in an atom is contained in the orbital wave function ‘Ψ’.
- The exact path of the electron can never be determined accurately. Therefore, we find only the probability of the electron at different points in space, around the nucleus of an atom.
- Significance of Ψ² : The probability of finding an electron at a point within an atom is proportional to the square of the electron wave function Ψ²
Ψ² is known as probability density function and is always positive.
From the value of Ψ² it is possible to predict the orbital, that is the space where electron will most probably be found around the nucleus.
![]()
Question 7.
What are the limitations of Bohr’s model of an atom? [Mar’ 13][AP 15,18]
Answer:
Limitations of Bohr’s model:
- Bohr’s theory’ could not explain the fine structure ( splitting of spectral lines ) of H-atom.
- Bohr’s theory fails to explain the spectra of multi-electron atoms like He, Li, Be etc.,
- Bohr’s theory could not explain the Zeeman effect (splitting of spectral lines in the presence of magnetic field)
- Bohr’s theory could not explain the Stark effect (splitting of spectral lines in the presence of electric field).
- Bohr’s theory could not explain the ability of atoms to form molecules by chemical bonds.
- Bohr’s theory could not explain de-Broglie’s dual nature of electrons.
Question 8.
What are the evidences in favour of dual behaviour of electron?
Answer:
Particle nature of electron :
Planck’s quantum theory and photoelectric effect support the particle nature of electrons. Also, according to Newton, light travels in the form of particles. This theory explains reflection and refraction of light.
Wave nature of electron :
Heisenberg’s uncertainty principle supports the wave nature of electron. Also, according to Huygens, light travels in the from of waves. This theory explains diffraction and interference of light.
Louis de-Broglie proposed the dual nature of matter. According to him, microscopic particles such as electrons have both wave-like & particle- properties.
Louis de-Broglie derived an equation to calculate the wavelength (λ) of the wave associated with a particle of mass m, moving with velocity v. It is given by
λ = \(\frac{h}{mv}=\frac{h}{p}\)
where ‘h’ is Planck’s constant and p is momentum of the particle.
Experimental Evidence :
The wave nature of electrons was verified experimentally by Davisson and Germer by carrying out diffraction experiments with a beam of fast moving electrons. The wave nature of electrons is utilised in the construction of electron microscope.
Question 9.
How are the quantum numbers n, l and ml arrived at? Explain the significance of these quantum numbers. [IPE’ 04, 06, 10, 10, 13, 14][AP, TS 15, 16, 17, 19]
Answer:
To explain the position of electron in the space around the nucleus, quantum numbers were proposed.
Various types of orbitals can be distinguished by their size, shape, and orientation. This can be done by means of quantum numbers.
The quantum numbers n, l, and ml are obtained when Schrodinger wave equation is solved for the wave function Ψ.
1) Principal quantum number :
i) It was proposed by Bohr.
ii) It is denoted by ‘n’.
iii) The values of ‘n’ are 1, 2, 3, 4 ……. These values correspond the shells K, L, M, N ………..
Significance :
The value of ‘n’ indicates (i) size of the orbit (rn) (ii) energy of the orbit (En)
• The maximum number of electrons in nth orbit = 2n².
2) Azimuthal quantum number :
- It was proposed by Sommerfeld.
- It is denoted by ‘l’.
- The values of ‘l’ are 0, 1, 2,….,(n – 1).
Significance : The value of ‘l’ indicates
i) shape of the subshell,
ii) angular momentum of electron
- The ‘l’ values 0, 1, 2, 3 correspond the sub shells s, p, d, f respectively.
- The shape of s-orbital is spherical, the shape of p-orbital is dumb-bell, the shape of d-orbital is double dumb-bell, the shape of f-orbital is ‘four fold dumb-bcIP.
- The angular momentum of electron is given by mvr = \(\sqrt{l(l+1)} \cdot \frac{\mathrm{h}}{2 \pi}\)
Hence, 7’ is also called ‘angular momentum quantum number’.
3) Magnetic quantum number :
- It was proposed by Lande.
- It is denoted by ‘m’(or) ml
- The values of ‘m’ range from “-l to +l” including ‘O’.
For a given value of ‘l’, the total number of ‘m values’ = 2l + 1.
Significance :
The value of ‘m’ indicates the ‘orientation’ of the orbital in space.
It explains the ZEEMAN effect.
4) Also, we have one more quantum number called Spin Quantum number(s), which was proposed by Uhlenbeck and Goudsmith. This quantum number signifies the spin (clockwise or anticlockwise) of the revolving electron.
Question 10.
Explain the dual behavior of matter. Discuss its significance to microscopic particles like electrons.
Answer:
de-Broglie proposed that micro particles like electrons exhibit both particle and wave like properties. Thus, electron should also have momentum as well as wave length.
1) According to Planck’s quantum theory of radiation, the energy of photon is given by
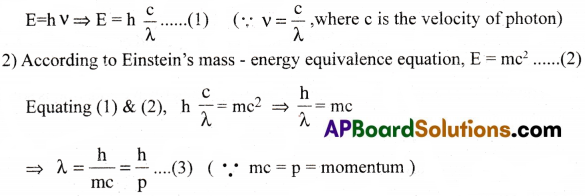
de Broglie hypothesized that equation (3) applicable to photons, can be extended to all micro particles like electrons, moving with high speeds.
Hence in (3), the velocity of photon (c) can be replaced by the velocity of microparticle (v).
∴ Equation (3) be re-written as λ = \(\frac{h}{p}=\frac{h}{mv}\) …….. (4)
This equation (4) is called de Broglie equation.
This λ is known as particle wave length (or) de Broglie wave length
Thus, the microparticle electron possess wave length λ, given by the above expression. Hence, electron is associated with wave nature.
Significance :
Equation (4) should hold true for all the moving material bodies.
In (4) ‘m’ is in the denominator. For macroscopic bodies like planets, the value of m is very large. Hence λ becomes very small, so that it is neglected.
But, microscopic particles like electrons possess very low masses. Hence, the value of λ is very significant.
Further, for an electron moving around the nucleus in circular path, two different types of waves of different wave lengths are possible.
One is due to constructive interference ( when nλ = 2πr )
and the other is due to destructive interference (nλ ≠ 2πr )
If the circumference of the electron orbit is an integral multiple of λ, then electron waves are said to be ‘in – phase’. Otherwise, they are ‘out of phase’.

(6) is nothing but the relation of Bohr’s quantization of angular momentum.
Thus, de Broglie’s theory and Bohr’s theory are in well agreement with each other, in the quantization of angular momentum.
Question 11.
What arc the various ranges of electromagnetic radiation? Explain the characteristics of electromagnetic radiation.
Answer:
According to Maxwell, “electromagnetic radiation” is made up of electromagnetic waves. They contain two components, one is varying electric field and the other is the varying magnetic field. These two fields are perpendicular to each other and are also perpendicular to the direction of propagation of wave.
Characteristics of electromagnetic radiations :
1) These radiations are produced by oscillating charged particles in a body.
2) The radiations can pass through vacuum also. So medium for transmission is not required.
3) The wavelength (λ) is the distance between two neighbouring crests or troughs of the wave.
Units of λ : cm, m, nm, Å
4) Frequency(v)of a wave is the number of waves which cross a particular point in one second.

Units of frequency: cps (cycles per sec) or Hz (hertz)
5) Velocity (c) of a wave is the distance travelled by the wave in one second.
Velocity = wavelength × frequency ⇒ c = λ × ν where c = 3 × 1010 cm/s.
Velocity of all electromagnetic radiations in space or in vaccum is the same.
6) Wave number (\(\overline{\mathrm{ν}}\)) is the number of wavelength per centimeter.
It is reciprocal of wavelength. \(\overline{\mathrm{ν}}\) = \(\frac{1}{\lambda}\) Units Wave number: cm-1
7) Amplitude (A) is the height of the crest or depth of trough of a wave.
It determines the intensity or brightness of the light.
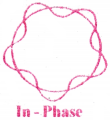
Various ranges of Electromagnetic spectrum:
(Wave length λ in meters and Frequency ν in Hz)
![]()
Question 12.
Define atomic orbital.Explain the shapes of s, p, d orbitals with the help of diagrams.
Answer:
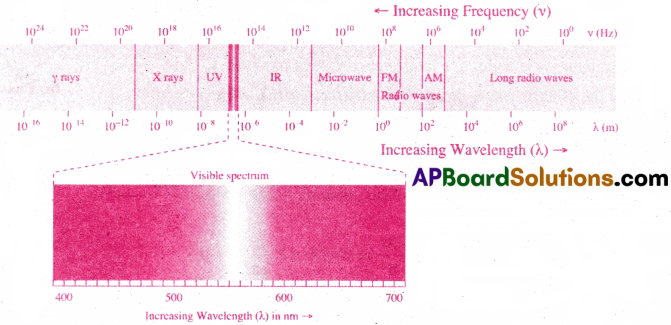
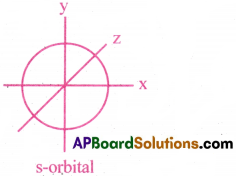
An atomic orbital is defined as the 3-dimensional space, around the nucleus in an atom, where the probability of finding an electron is maximum (95%).
I) Shape of s-orbital :
- The shape of s-orbital is spherical.
- As the value of ‘n’ increases, the size of the orbital increases.
That is, 1s < 2s < 3s < - s-orbital does not possess any directional property.
- The number of nodal planes for s-orbital is ‘zero’. That is, l =0.
- Various possible quantum numbers for s-orbitals are :
n = 1, 2, 3 ….; l = 0, m = 0.
II) Shape of p-orbitals :
- The shape of p-orbital is dumb-bell with 2 lobes.
- The p-subshell contains 3 sub-orbitals.They are px, py, pz degenerate orbitals.
- All the 3 sub-orbitals are mutually perpendicular.
- Each p-orbital has 1 nodal plane. That is, l = 1.
- Various possible quantum numbers for p-orbital are: n = 2,3,4,….; l = 1; m = -1, 0, 1
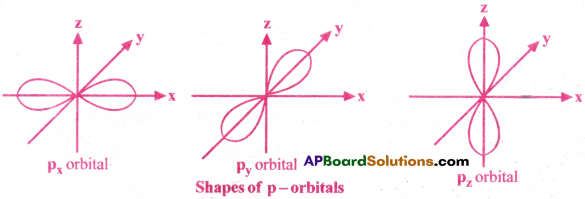
III) Shape of d-orbitals :
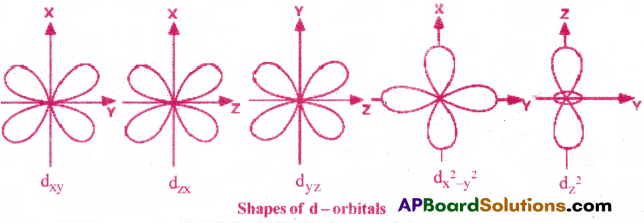
- The shape of d-orbital is double dumb-bell with 4 lobes.
- The d -subshell contains 5 sub-orbitals. They are dxy, dyz, dzx, dx²-y² and dz²
- The shapes of first 3 orbitals are similar and their lobes are in between their axes.
- Each d-orbital has 2 nodal planes.That is, l = 2
- Various possible quantum numbers for d-orbital are: n = 3, 4, 5 ; l = 2; m = -2, -1, 0, 1, 2.
Question 13.
Explain diagrammatically the boundary surfaces for three 2p orbitals and five 3d orbitals.
Answer:
1) Shape of 2p-orbital :
Shape Of p-orbital is dumb-bell. There are three 2p- orbitals. They are 2px, 2py and 2pz orbitals. Each orbital has two lobes. These lobes are oriented along their respective axes. Each p orbital has one nodal plane.
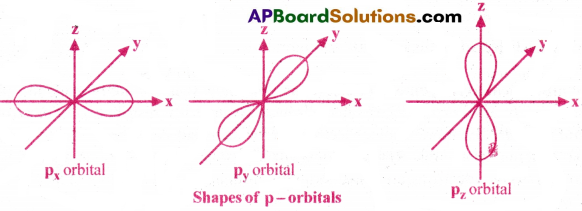
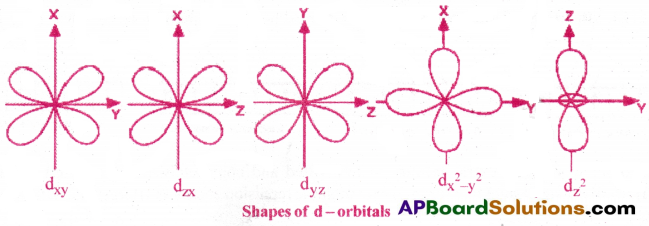
2) Shape of 3d- orbital :
d-orbital has double dum-bell shape. There are five d- orbitals. They are dxy, dyz, dzx, dx²-y² and dz². Each d orbital has 4 lobes. In dxy orbital, the lobes are placed in between the x and y axes. Similar is the case with other orbitals dyz, dzx. In dx²-y² orbital, the lobes lie along x and y – axes. Each d orbital has two nodal planes. In dz² orbital, two lobes lie along z axis and there is a ring of electron cloud around the centre.
Question 14.
Illustrate the reasons for the stability of completely filled and half-filled subshells.
Answer:
The completely filled and half-filled orbitals give stability to the atoms due to following reasons.
- Symmetrical arrangement of electrons in sub-shells.
- More number of exchange energies.
These can be illustrated by taking the electronic configurations of Chromium and Copper.
The expected configurations for Cr and Cu are:
Cr(Z = 24) : [Ar] 4s²3d4
u(Z = 29): [Ar] 4s²3d9
The two sub-shells 4s and 3d differ very’ slightly in their energies. When an electron shifts from a sub-shell of lower energy (4s) to (3d) the electronic configuration of Cr and Cu changes as follows:
Cr(Z = 24) : [Ar]4s¹3d5
Cu(Z = 29) : [Ar]4s¹3d10
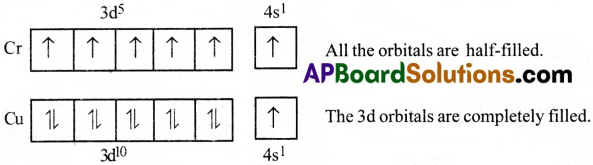
Thus, Cr gets half filled 3d – shell electronic configuration and
Cu gets full filled 3d-shell electronic configuration.
1) Symmetrical arrangement of electrons :
We know that symmetry leads to stability. The completely filled or half-filled subshells have symmetrical distribution of electrons in them and are therefore more stable. Electrons in the same subshell (here 3d) have equal energy but different spatial distribution. Consequently, their shielding of one another is relatively small and the electrons are more strongly attracted by the nucleus. Hence the atom gets more stability.
2) Exchange energies :
The shifting of electrons from one orbital to another orbital in the same subshell is called exchange.The energy released due to this exchange is called exchange energy.
When two or more electrons with the same spin in the degenerate orbitals, exchange their positions then some energy is released. Then the energy of the atom decreases and it becomes more stable. The more the number of ‘exchange pair’ of electrons, the greater would be the decrease of energy and hence the greater is the stability of the atom.
Ex : In 3d5 configuration, the electron pair exchanges can happen in 10 ways.
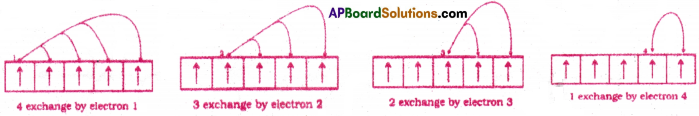
In other words, the extra stability of half-filled and completely filled subshell is due to (i) relatively small shielding (ii) smaller coulombic repulsion energy (iii) larger exchange energy.
Question 15.
Explain emission and absorption spectra. Discuss the general description of the spectra in hydrogen atom.
Answer:
Emission spectrum:
- Emission spectrum is formed due to emission of radiation in quanta.
- It is produced when electrons jump from higher orbits to lower orbits.
- It consists of bright lines on a dark back ground.
Absorption spectrum:
- Absorption spectrum is formed due to absorption of energy in quanta.
- It is produced when electrons jump from lower orbits to higher orbits.
- It consists of dark lines on a bright back ground.
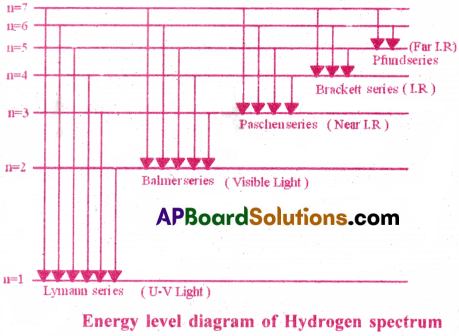
Hydrogen Spectrum :
When an electric discharge is passed through hydrogen gas at low pressure, a bright light is emitted by hydrogen atoms.
When this light is passed through a prism, a discontinuous line spectrum of several sharp, distinct lines is produced. It is called hydrogen emission spectrum.
The transmission of energy of electrons from any higher orbit to
- n = 1 produces spectral lines in the UV region. This is named as Lyman series.
- n = 2 produces spectral lines in the visible region. This is named as Balmer series.
- n = 3, 4, 5 produces spectral lines in the IR region. These are named as Paschen, Brackett and Pfund series respectively
The energy level diagram is shown below
Multiple Choice Questions
Question 1.
Calculate the number neutrons and electrons in
1) 25, 45
2) 25, 35
3) 35, 45
4) 30, 40
Answer:
3) 35, 45
Solution:
For 8035Br, Atomic mass number A = 80 and
Atomic number Z = 35.
For a neutral atom,
No. of protons = No. of electrons = Z = 35
No.of neutrons = A – Z = 80 – 35 = 45
![]()
Question 2.
The number of electrons, protons, and neutrons in a species are equal to 18,16 and 16 respectively. Then the proper symbol to the species is
![]()
Answer:
2
Solution:
Given No.of electrons = 18,
Number of protons =16, No.of neutrons = 16
The atomic number(Z) = No. of protons =16.
∴ The element is sulphur(S).
Atomic mass number(A)
= No.of protons + No.of neutrons = 16 + 16 = 32
The number of protons is not equal to electrons.
∴ The species is riot neutral.
It is an anion with charge equal to excess number of electrons = 18 – 16 = 2.
∴ The Symbol is 3216S2-
Question 3.
The Vividh Bharati station of AH India Radio, Delhi broadcasts on a frequency of l,368KHz(Kilo Hertz). Calculate the wavelength of the electromagnetic radiation emitted by transmitter. Which part of the electromagnetic spectrum does it belong to?
1) 215.3 m, Radio waves
2) 217.3 m, Radio waves
3) 216.3 m, Radio waves
4) 219.3 rh, Radio waves
Answer:
4) 219.3 rh, Radio waves
Solution:
Frequency ν = 1,368 KHz = 1368 × 10³ s-1
Velocity of radiation c = 3 × 108 m.s-1
Formula :
Wavelength (λ) = \(\frac{c}{ν}\)
= \(\frac{3 \times 10^8}{1368 \times 10^3}\) = 219.3m
This wave length belongs to Radio waves.
Question 4.
The wavelength range of the visible spectrum extends from violet (400nm) to red (750nm). Express these wavelengths in frequencies (Hz).(1nm = 10-9m)
1) 6.50 × 1014Hz; 3.00 × 1014Hz
2) 7.50 × 1014Hz; 4.00 × 1014Hz
3) 8.50 × 1014Hz; 4.00 × 1014Hz
4) 6.05 × 1014Hz; 3.00 × 1014Hz
Answer:
2) 7.50 × 1014Hz; 4.00 × 1014Hz
Solution:
Frequency of violet

Question 5.
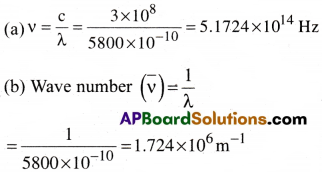
Calculate the frequency (v) and wave number (\(\overline{\mathrm{ν}}\)) of the yellow light of wave length 5800Å, emitted from Sodium lamp.
1) 5.1724 × 1014Hz; 1.724 × 106m-1
2) 5.1824 × 1014Hz; 1.624 × 106m-1
3) 6.1724 × 1014Hz; 1.724 × 106m-1
4) 4.1724 × 1014Hz; 2.724 × 106m-1
Answer:
1) 5.1724 × 1014Hz; 1.724 × 106m-1
Solution:
Velocity of light c = 3 × 108m /s
wavelength of yellow light λ = 5800Å = 5800 ×10-10m
Question 6.
Calculate energy of one mole of photons of radiation whose frequency is 5 × 1014 Hz.
1) 196.51 kJmol-1
2) 199.51 kJmol-1
3) 190.51 kJ mol-1
4) 191.51 kJmol-1
Answer:
2) 199.51 kJmol-1
Solution:
Given Frequency v = 5 × 1014 s-1
We know h = 6.626 × 10-34 Js;
Energy (E) of one photon is E = hν;
E = (6.626 × 10-34 Js) × (5 × 1014s-1)
= 3.313 × 10-19 J
Energy of one mole of photons
= (3.313 × 10-19J) × (6.023 × 1023 mol-1)
= 199.51 kJ mol-1.
![]()
Question 7.
A 100 watt bulb emits monochromatic light of wavelength 400nm. Calculate the number of photons emitted per second by the bulb.
1) 2.312 × 1019S-1
2) 2.111 × 1020S-1
3) 2.012 × 1018S-1
4) 2.012 × 1020S-1
Answer:
4) 2.012 × 1020S-1
Solution:
Power of the bulb = 100watt = 100Js-1.
Energy of one photon E = hν = hc/λ
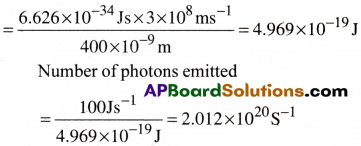
Question 8.
When electromagnetic radiation of wavelength 300nm falls on the surface of sodium, electrons are emitted with a kinetic energy of 1.68 × 105J mol-1. What is the minimum energy needed to remove an electron from sodium? What is the maximum wavelength that will cause a photoelectron to be emitted?
1) 3.84 × 10-19J, 517 nm
2) 4.84 × 10-19J, 519 nm
3) 5.84 × 10-19J, 617 nm
4) 3.84 × 10-19J, 717 nm
Answer:
1) 3.84 × 10-19J, 517 nm
Solution:
Given Wavelength(λ) = 300nm = 300 × 10-19m

The energy of one mole of photons
= 6.626 × 10-19J × 6.022 × 1023mol-1.
= 3.99 × 105Jmol-1.
The minimum energy needed to remove one mole of electrons from sodium. hν0 = hν – KE
= (3.99 – 1.68)105Jmol-1 = 2.31 × 105Jmol-1
The minimum energy for one electron
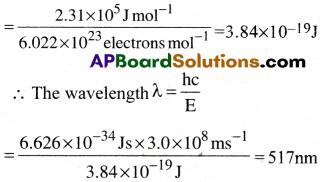
Wavelength 517 nm corresponds to green light.
Question 9.
The threshold frequency v0 for a metal is 7.0 × 1014 s-1. Calculate the kinetic energy of an electron emitted when radiation of frequency v = 1.0 × 1015 s-1 hits the metal.
1) 1.555 × 10-19 J
2) 1.987 × 10-19 J
3) 1.987 × 10-17 J
4) 1.787 × 10-15 J.
Answer:
2) 1.987 × 10-19 J
Solution:
Threshold Frequency ν0 = 7.0 × 1014 sec-1
Frequency of radiation ν
= 1.0 × 1015sec-1 = 10 × 1014 sec-1
Plancks constant h = 6.626 × 10-34 Js
Formula: KE = h (ν – ν0)
= 6.626 × 10-34(10 × 1014 – 7 × 1014)
= 1.987 × 10-19J.
Question 10.
What are the frequency and wavelength of a photon emitted during a transition from n = 5 state to the n = 2 state in the hydrogen atom?
1) 0.6914 × 1016 Hz; 4.429 × 10-8m
2) 0.6914 × 1015 Hz; 3.329 × 10-7m
3) 0.6814 × 1017 Hz; 4.329 × 10-9m
4) 0.6914 × 1015 Hz; 4.329 × 10-7m
Answer:
4) 0.6914 × 1015 Hz; 4.329 × 10-7m
Solution:
Let n1 = 2, n2 = 5; we take R = 1.1 × 10–m-1
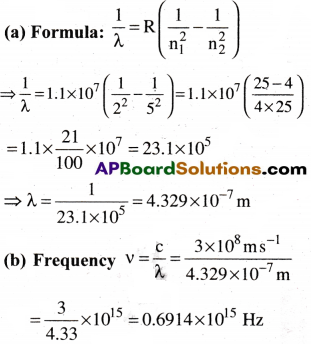
Question 11.
Calculate the energy associated with the first orbit of He+. What is the radius of this orbit?
1) -8.72 × 10-17J, 0.2745Å
2) -7.92 × 10-16J, 0.2645Å
3) -8.72 × 10-18J, 0.2645Å
4) -7.52 × 10-14J, 0.2545Å
Answer:
3) -8.72 × 10-18J, 0.2645Å
Solution:
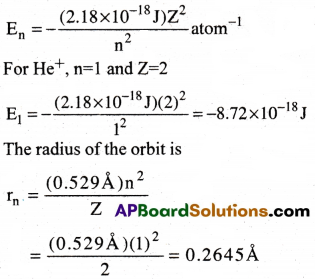
Question 12.
What will be the wavelength of a ball of mass 0.1 Kg moving with a velocity of 10ms-1?
1) 6.6469 × 10-34m
2) 6.62 69 × 10-34m
3) 6.6469 × 10-32m
4) 6.6969 × 10-32m
Answer:
2) 6.62 69 × 10-34m
Solution:
Given Mass m = 0.1kg ; Velocity = 10ms-1
We know h = 6.626 × 10-34Js

![]()
Question 13.
The mass of an electron is 9.1 × 10-31kg. If its K.E is 3.0 × 10-25J. Calculate its wave length.
1) 896.7 nm
2) 859.7 nm
3) 876.7 nm
4) 866.7 nm
Answer:
1) 896.7 nm
Solution:
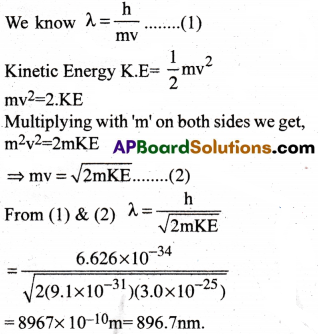
Question 14.
Calculate the mass of a photon with wavelength 3.6 A
1) 6.137 × 10-32Kg
2) 6.135 × 10-11Kg
3) 6.135 × 10-33Kg
4)6.135 × 10-27Kg
Answer:
3) 6.135 × 10-33Kg
Solution:
Wavelength λ = 3.6 Å = 3.6 × 10-10 m
Velocity of photon = Velocity of light
v = 3 × 108 m.sec-1
Planck’s constant h = 6.626 × 10-34 Js
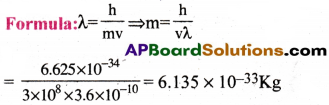
Question 15.
A microscope using suitable photons is employed to locate an electron in an atom within a distance of 0.1 A. What is the uncertainty involved in the measurement of its velocity?
1) 6.79 × 108ms-1
2) 5.79 × 106ms-1
3) 5.79 × 108ms-1
4) 6.79 × 106ms-1
Answer:
2) 5.79 × 106ms-1
Solution:
From Heisenberg uncertainty principle,
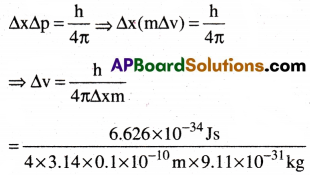
= 0.579 × 107 ms-1 =5.79 × 106 ms-1 (∵ 1J = lkgm²s-1)
Question 16.
A golf ball has a mass of 40g, and a speed of 45m/s. If the speed can be measured with in accuracy of 2%. Calculate the uncertainty in the position.
1) 1.46 × 10-36m
2) 1.49 × 10-33m
3) 1.42 × 10-33m
4) 1.46 × 10-33m
Answer:
4) 1.46 × 10-33m
Solution:
Given mass m = 40g =40 × 10-3kg
The uncertainty in the speed is
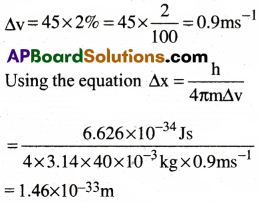
Question 17.
The number of protons, neutrons and electrons in 17571Lu respectively are
1) 71,104 and 71
2) 104, 71 and 71
3) 71,71 and 104
4) 175, 104 and 71
Answer:
1) 71,104 and 71
![]()
Question 18.
Two atoms are said to be isobars, if
1) they have same atomic number but different mass number.
2) they have same number of electrons but different number of neutrons.
3) they have same number of neutrons but different number of electrons.
4) sum of the number of protons and neutrons is same but the number of protons is different.
Answer:
4) sum of the number of protons and neutrons is same but the number of protons is different.
Question 19.
Which of the following properties of atom could be explained correctly by Thomson Model of atom?
1) Overall neutrality of atom.
2) Spectra of hydrogen atom.
3) Position of electrons, protons and neutrons in atom.
4) Stability of atom.
Answer:
1) Overall neutrality of atom.
Question 20.
Which of the following conclusions could not be derived from Rutherford’s a-particle scattering experiment?
1) Most of the space in the atom is empty.
2) The radius of the atom is about 10_1° m while that of nucleus is 10-15 m.
3) Electrons move in a circular path of fixed energy called orbits.
4) Electrons and the nucleus are held together by electrostatic forces of attraction.
Answer:
3) Electrons move in a circular path of fixed energy called orbits.
Question 21.
Which of the following statements about the electron is incorrect?
1) It is a negatively charged particle
2) The mass of electron is equal to the mass of neutron
3) It is a basic constituent of all atoms
4) It is a constituent of cathode rays
Answer:
2) The mass of electron is equal to the mass of neutron
Question 22.
Which of the following is responsible to rule out the existence of definite paths or trajectories of electrons?
1) Pauli’s exclusion principle
2) Heisenberg’s uncertainty principle
3) Hund’s rule of maximum multiplicity
4) Aufbau principle
Answer:
2) Heisenberg’s uncertainty principle
Question 23.
Orbital angular momentum depends on ……….
1) 1
2) n and l
3) n and m
4) m and s
Answer:
1) 1
Question 24.
Total number of orbitals associated with third shell will be
1) 2
2) 4
3) 9
4) 3
Answer:
3) 9
![]()
Question 25.
A particular station of All India Radio, New Delhi, broadcasts on a frequency of l,368KHz (kilohertz). The wavelength of the electro magnetic radiation emitted by the transmitter is
[speed of light, c = 3 × 108 ms–]
1)21.92 cm
2) 219.3 m
3) 219.2 m
4) 2192 m
Answer:
2) 219.3 m
Question 26.
Which of the following series of transition in the spectrum of hydrogen atom falls in visible region?
1) Brackett series
2) Lyman series
3) Balmer series
4) Paschen series
Answer:
3) Balmer series
Question 27.
Number of angular nodes for 4d orbital is …………
1) 4
2) 3
3) 2
4) 1
Answer:
3) 2
Question 28.
The number of radial nodes for 3p orbital is ………….
1) 3
2) 4
3) 2
4) 1
Answer:
4) 1
Question 29.
Orbital having 3 angular nodes and 3 total nodes is ………….
1) 5p
2) 3d
3) 4f
4) 6d
Answer:
3) 4f
![]()
Question 30.
4d, 5p, 5f and 6p orbitals are arranged in the order of decreasing energy. The correct option in the order of decreasing energy
1) 5f > 6p > 4d > 5p
2) 5f > 6p > 5p > 4d
3) 6p > 5f > 5p > 4d
4) 6p > 5f > 4d > 5p
Answer:
2) 5f > 6p > 5p > 4d
Question 31.
Which of the following options does not represent ground state electronic configuration of an atom?
1) 1s² 2s² 2p6 3s² 3p6 3d8 4s²
2) 1s² 2s² 2p6 3s² 3p6 3d9 4s²
3) 1s² 2s² 2p6 3s² 3p6 3d10 4s¹
4) 1s² 2s² 2p6 3s² 3p6 3d5 4s¹
Answer:
2) 1s² 2s² 2p6 3s² 3p6 3d9 4s²
Question 32.
The pair of ions having same electronic configuration is ……………
1) Cr3+, Fe3+
2) Fe3+, Mn3+
3) Fe3+, CO3+
4) Sc3+, Cr3+
Answer:
2) Fe3+, Mn3+
Question 33.
From the following pairs of ions which one is not an iso-electronic pair?
1) Fe2+, Mn2+
2) O2-, F–
3) Na+, Mg2+
4) M2+, Fe3+
Answer:
1) Fe2+, Mn2+
Question 34.
In hydrogen atom, the de Broglie wavelength of an electron in the second Bohr orbit is
[Given that Bohr radius, a0 = 52.9 pm]
1) 211.6 pm
2) 211.6 πpm
3) 52.9 πpm
4) 105.8 pm
Answer:
2) 211.6 πpm
Question 35.
If travelling at same speeds, which of the following matter waves have the shortest wavelength?
1) Electron
2) Alpha particle (He2+)
3) Neutron
4) Proton
Answer:
2) Alpha particle (He2+)
Question 36.
Chlorine exists in two isotopic forms, Cl-37 and Cl-35 but its atomic mass is 35.5. This indicates the ratio of Cl-37 and Cl-35 is approximately
1) 1 : 2
2) 1 : 1
3) 1 : 3
4) 3 : 1
Answer:
3) 1 : 3
![]()
Question 37.
Which one is a wrong statement?
1) Total orbital angular momentum of electron in s-orbital is equal to zero
2) An orbital is designated by three quantum numbers while an electron in an atoms is designated by four quantum numbers
3) The value of m for dz² is zero
4) The electronic configuration of N atom
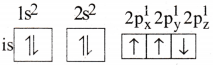
Answer:
4) The electronic configuration of N atom
