Solving AP 10th Class Physical Science Model Papers and AP 10th Class Physical Science Question Paper April 2022 regularly is an effective strategy for time management during exams.
AP SSC Physical Science Question Paper April 2022 with Solutions
Time: 2 Hours
Maximum Marks: 50
Instructions:
- The question paper consists of 4 sections and 17 questions.
- Internal choice is available only for Q.No.12 in section III and for all the questions in section IV.
- In 2 hours, 15 minutes is allotted to read the question paper.
- All answers shall be written in the answer booklet only.
- Answers shall be written neatly and legibly.
Section-I
(8 × 1 = 8 Marks)
Note:
- Answer ALL the questions.
- Each question carries 1 mark.
Question 1.
Statement P: An optically denser medium may not possess greater mass density.
Statement Q: Kerosene with a high refractive index is optically denser than water.
(A) Both P and Q are correct.
(B) P-correct, Q-wrong.
(C) P-wrong, Q-correct.
(D) Both P and Q are wrong.
Answer:
(A) Both P and Q are correct.
Question 2.
The value of least distance of distinct vision of the healthy human beings _________
Answer:
25 cm
Question 3.
Which rule is violated in the electronic configuration 1s02s22p4?
Answer:
Aufbau
![]()
Question 4.
Which element does not have 8 electrons in its valency shell among noble gases?
Answer:
He or Helium
Question 5.
A solution turns red litmus into blue, its pH value is _________
(A) 1
(B) 4
(C) 5
(D) 10
Answer:
(D) 10
Question 6.
Write the name of the device that converts mechanical energy into electrical energy.
Answer:
Electric Generator (or) Dynamo
Question 7.
Convert the boiling point of water at STP into Kelvin.
Answer:
373K
Question 8.
Who produced an organic compound urea in the laboratory by heating an inorganic salt ammonium cyanate and silver chloride?
Answer:
F. Wohler
Section-II
(3 × 2 = 6 Marks)
Note:
- Answer ALL the questions.
- Each question carries 2 marks.
Question 9.
An element X belongs to the 3rd period and group 2 of the modern periodic table. Predict the number of valence electrons and write.
Answer:
As the element belongs to group II, the valency electrons are 2.
Question 10.
Mention the daily life applications of the thermite process.
Answer:
- To join railings of railway tracks.
- To join cracked machine parts.
![]()
Question 11.
Your friend is asked to differentiate between evaporation and boiling. What questions would you ask to make him know the differences between evaporation and boiling?
Answer:
- Why does evaporation take place at any temperature?
- Why does boiling take place at a constant temperature?
Section-III
(3 × 4 = 12 Marks)
Note:
- Answer ALL the questions.
- Each question carries 4 marks.
Question 12.
Draw any one of the following diagrams:
(A) Draw a neat diagram of the Reverberatory furnace.
(B)
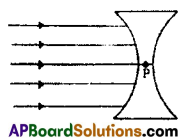
Draw lines of refraction of the above diagram and locate the point of the image.
Answer:
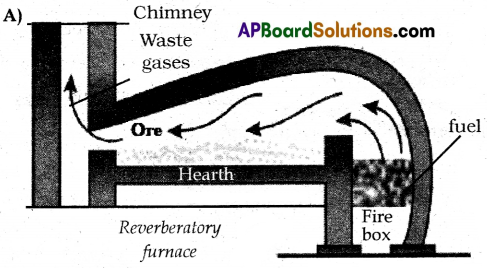
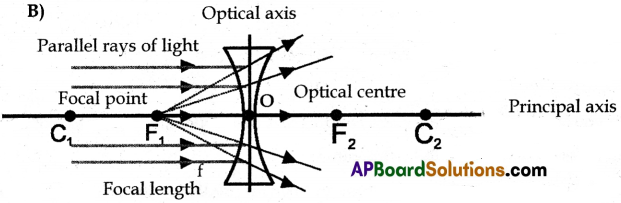
Question 13.
Observe the table and answer the following questions:
| Solution | A | B | C | D | E | F | G | H |
| pH Value | 8 | 2 | 6 | 7 | 13 | 1 | 9 | 12 |
(i) Which solution is neutral?
(ii) Which solutions are strong acids?
(iii) Which solutions are strong bases?
(iv) Which solutions are weak bases?
Answer:
(i) D
(ii) F, B
(iii) H, E
(iv) A, G
Question 14.
Appreciate the role of optical fibers in cell phone communications.
Answer:
We cannot imagine present-day technology without optical fibers. They work on the principle of Total Internal Reflection (TIR). All the information is passed through these fibers. The clarity of the signals transmitted in this way is much better than in other conventional methods.
Section-IV
(3 × 8 = 24 Marks)
Note:
- Answer ALL the questions.
- Each question carries 8 marks.
- Each question has an internal choice.
Question 15.
(A) What is Myopia? Explain the correction of the defect Myopia.
(OR)
(B) How do the following properties change in a group and period?
(i) Atomic radius
(ii) Ionization energy
(iii) Electron affinity
(iv) Electronegativity
Answer:
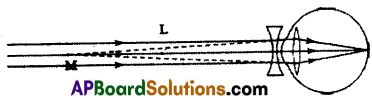
(A) (i) Some people cannot see the object at long distances but can see near objects. This type of eye defect in vision is called myopia.
(ii) In people suffering from myopia the rays coming from distant objects after refraction through the eye lens form an image before the retina.
(iii) By using a biconcave lens we can rectify myopia.
(iv)
(OR)
(i) (a) Atomic radius increases in groups from top to bottom.
(b) Atomic radius decreases in periods from left to right.
(ii) (a) Ionization energy decreases in groups from top to bottom.
(b) Ionization energy increases.
(iii) (a) Electron affinity decreases in groups from top to bottom.
(b) Electron affinity increases in periods from left to right.
(iv) (a) Electronegativity decreases in groups from top to bottom.
(b) Electronegativity increases in periods from left to right.
![]()
Question 16.
(A) Suggest an experiment to find the specific heat of a solid.
(OR)
(B) How do you verify that the resistance of a conductor is proportional to the length of the conductor for constant cross-sectional area and temperature?
Answer:
(A) Aim: To find the specific heat of a solid.
Material required: Calorimeter, thermometer stirrer, water steam heater, lead shots, and wood box.
Procedure: Measure the mass of the calorimeter along with the stirrer, the mass of the calorimeter = m1
(i) Fill one-third of the volume of the calorimeter with water and measure its mass and temperature.
Mass of calorimeter plus water = m2
(ii) Mass of water = m2 – m1
The initial temperature of the calorimeter = T1° C = Initial temperature of the water
(iii) Take a few lead shots place them in hot water and heat up to 100°C.
Let this temperature be T2°C.
(iv) Transfer the lead shots quickly into the water in a calorimeter. Stir it well and note the final temperature of T3°C.
(v) Measure the mass of lead shots water and calorie meter = m3
Mass of lead shots = m3 – m2
The formula of the method of mixtures:
Heat lost by solid (Lead-shots) = Heat gained by calorimeter + Heat gained by water
⇒ (m3 – m2) SL (T2 – T3) = m1SC
(T3 – T1) + (m2 – m1) SW (T3 – T1)
Specific Heat of solid (SL) = \(\frac{\left[\mathrm{m}_1 \mathrm{~S}_{\mathrm{C}}+\left(\mathrm{m}_2-\mathrm{m}_1\right) \mathrm{S}_{\mathrm{W}}\right]\left(\mathrm{T}_3-\mathrm{T}_1\right)}{\left(\mathrm{m}_3-\mathrm{m}_2\right)\left(\mathrm{T}_2-\mathrm{T}_3\right)}\)
(OR)
(B) (i) Collect iron spokes of different lengths with the same cross-sectional area like copper, aluminum, etc.
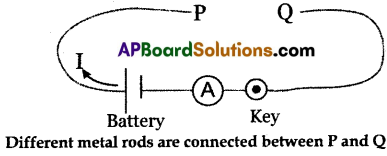
(ii) Make a circuit as shown in the figure.
(iii) Connect one of the iron spokes, say 10 cm in length, between P and Q, and switch on the circuit.
(iv) Measure the value of the current using the ammeter connected to the circuit and note the values.
(v) Repeat this procedure for other lengths of the iron spokes.
(vi) Note corresponding values of currents.
(vii) We notice that the current decreases with an increase in the length of the spoke.
(viii) Thus, the resistance of each spoke increases in the length for a constant potential difference.
(ix) From this activity, we can conclude that the resistance (R) of a conductor is directly proportional to its length (l) for a constant potential difference,
i.e., R ∝ l (at constant temperature and cross-sectional area).
Question 17.
(A) Explain the significance of three quantum numbers in predicting the positions of an electron in an atom.
(OR)
(B) What are the important postulates in VSEPR theory?
Answer:
(A) Each electron in an atom is described by a set of three quantum numbers n, l, and ml. These numbers indicate the probability of finding the electron in the space around the nucleus.
Fill one-third of the volume of the calorimeter with water and measure its mass and temperature. Mass of
(a) Principal quantum number (n):
- It was introduced by Niels Bohr.
- The principal quantum number explains the size and energy of the orbitals.
- These orbitals are called energy levels or shells. It is represented by ‘n’, where n = 1, 2, 3,…… etc.
- As ‘n’ increases, the shells become larger and the electrons in those orbitals are farther from the nucleus.
- As ‘n’ increases the energy of the shells also increases.
- The number of electrons in a shell is limited to 2n2.
- The shells are denoted by the letters K, L, M, N,…… etc.
| Shells | K | L | M | N | O |
| n | 1 | 2 | 3 | 4 | 5 |
(b) Orbital quantum number (l) (Or) Angular momentum quantum number (l):
- It was introduced by Sommerfeld.
- This quantum number defines the shape of the orbital occupied by the electron and the orbital angular momentum of the electron in motion.
- Due to this fact, it is also called angular momentum quantum number. It is represented by ‘l’.
- ‘l’ has integer values from 0 to (n – 1) for each value of n.
- Each value of l is related to the shape of orbitals in the space around the nucleus.
| l | 0 | 1 | 2 | 3 |
| Name of the Orbital | s | p | d | f |
- These orbitals (s, p, d, f, ….) are generally called sub-shells.
- Orbitals have the same value of ‘n’ but different values of ‘l’.
- The quantum number ‘l’ also governs the degree to which the electron is attached to the nucleus.
- The larger the value of ‘l’, the smaller is bond with which it is attached to the nucleus.
(c) Magnetic orbital quantum number (m1):
- To explain the Zeeman effect and Stark effect, ‘magnetic orbital quantum number’ is introduced by Lande.
- The orientation of the orbital with the external magnetic field determines the magnetic orbital quantum number (m1).
- m1 has integer values between -l and +l including zero. Thus for a certain value of ‘l’ there are (2l + 1) integer values for m1. They are -l, -l + 1,….., 0, l – 1, 1.
- These values describe the orientation of the orbital in space relative to the other orbitals in the atom when it is kept in a strong magnetic field.
- When l = 0, (2l + 1) = 1, there is only one value of m1.
- When l = 1, (2l + 1) = 3, that means m1 has three values namely, -1, 0, +1.
- The orientations of these three are along the x, v, and z axes. These are labeled as px, py, and pz.
- Orbitals in the sub-shell belonging to the same shell possess the same energy with different orientations, these are called degenerate orbitals.
Spin quantum number (ms):
- It was introduced by Uhlenbeck and Goldsmith.
- It is denoted by the letter ‘ms’.
- This quantum number refers to the two possible orientations of the spin of an electron, one clockwise (↑) and the other anticlockwise (↓) spin.
- The spin motion of the electrons is represented by \(+\frac{1}{2}\) and \(-\frac{1}{2}\).
(OR)
(B) To explain the bond angle in molecules with three or more than three atoms with all atoms attached to a central atom through covalent bond Valence – Shell – Electron-Pair – Repulsion Theory was proposed by Sidgwick and Powell and further improved by Gillespie and Nyholm.
![]()
Important Postulates:
- VSEPR Theory considers electrons in the valence shell which are in covalent bonds and lone pair as charge clouds that repel one another and stay as far apart as possible. This is the reason why molecule gets specific shapes.
- The total number of electron pairs in the die valence shell as covalent bonds and lone pairs in the central atom will help us to predict the arrangement of those pairs around the nucleus of the central atom and from that the shape of the molecule.
- Lone pairs occupy more space around the central atom than bond pairs. A lone pair means unshared electron pairs or non-bond pairs are shared by two nuclei.
- The presence of lone pairs on the central atom causes slight distortion of the bond angles from the expected regular shape. If the angle between the lone pair and the bond pair increases at the central atom due to more repulsion, the actual bond angles between atoms must be decreased.
| No. of Bond Pairs | No. of Lone Pairs | Shape | Bond Angle | Example |
| 2 | 0 | Linear | 180° | BeCl2 |
| 3 | 0 | Trigonal Planar | 120° | BCl3, BF3 |
| 4 | 0 | Tetrahedral | 109°28′ | CH4 |
| 3 | 1 | Pyramidal | 107°48′ | NH3, PCl3 |
| 2 | 2 | Angular or Bent | 104°28′ | H2O |