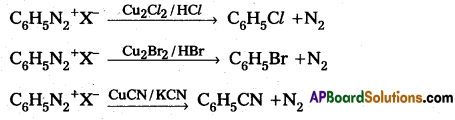Access to a variety of AP Inter 2nd Year Chemistry Model Papers and AP Inter 2nd Year Chemistry Question Paper March 2018 helps students overcome exam anxiety by fostering familiarity. question patterns.
AP Inter 2nd Year Chemistry Question Paper March 2018
Note : Read the following instructions carefully.
- Answer all questions of Section – ‘A’. Answer any six questions in Section – ‘B’ and any two questions in Section – ‘C’.
- In Section – A, questions from Sr. Nos. 1 to 10 are of “Very Short Answer Type”. Each question carries two marks. Every answer may be limited to 2 or 3 sentences. Answer all these questions at one place in the same order.
- In Section -”’8′, questions from Sr. Nos. 11 to 18 are of “Short Answer Type”. Each question carries four marks. Every answer may be limited to 75 words.
- In Section – ‘C’, questions from Sr. Nos. 19 to 21 are of “Long Answer Type”. Each question carries eight marks. Every answer may be limited to 300 words.
- Draw labelled diagrams wherever necessary for questions in Section – ‘B’ and ‘C’.
Section – A
Note : Answer all the questions.
Question 1.
Define Osmotic Pressure.
Answer:
Osmotic pressure : The pressure required to prevent the in flow of solvent molecules into the solution when these are separated by semi-permeable membrane.
Question 2.
What are antibiotics ? Give example.
Answer:
Antibiotics : The chemical substances produced by micro organisms and inhibit the growth or destroy micro-organisms are called antibiotics.
(Or)
The substance produced totally or partly by chemical synthesis which in low concentration inhibits the growth (or) destroy micro organism by intervening in their metabolic process are called antibiotics. E.g. : Penicillin, Chloramphenicol etc.
Question 3.
Write the difference between a soap and a synthetic detergent.
Answer:
- Generally soaps are sodium or potassium salts of long chain fattyacids.
- Synthetic detergents are cleansing agents having all the properties of soaps and donot contain any soap.
- Soaps donot work in hard water but synthetic detergents can be used both in soft and hard water as they give foam even in hard water. Some of the detergents give foam even in ice cold water.
Question 4.
State Faraday’s first law of electrolysis.
Answer:
The amount of chemical reaction which occurs at any electrode during electrolysis is proportional to the quantity of current passing through the electrolyte.
(Or)
The mass of the substance deposited at an electrode during the electrolysis of electrolyte is directly proportional to quantity of electricity passed through it.
m ∝ Q; m ∝ c × t
m = ect; m = \(\frac{\text { Ect }}{96,500}\)
e = electrochemical equivalent
t = time in seconds
c = Current in amperes
E = Chemical equivalent
![]()
Question 5.
Give the composition of the following alloys :
a) Brass
b) German Silver.
Answer:
a) Composition of Brass: 60 – 80% Cu, 20 – 40% Zn.
b) Composition of German silver : 50 – 60% Cu, 10 – 30% Ni, 20 – 30% Zn.
Question 6.
List out any two uses of Neon.
Answer:
Uses of Ne :
- Ne is used in discharge tubes and fluor escent bulbs for advertisement display purpose.
- ‘Ne’ – bulbs are used in botanical gardens and in green houses.
Question 7.
Write the structure of XeF4.
Answer:
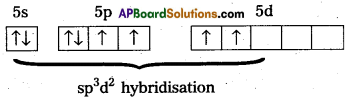
Structure of XeF4 :
1) Central atom in XeF4 is ‘Xe’.
2) Xe undergoes sp3d2 hybridisation in it’s 2nd excited, state.
3) Shape of the molecule is square planar with bond angle 90° and bond length 1.95A.
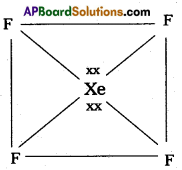
4) Xe – forms four σ – bonds by the overlap of sp3d2 – 2Pz(F) orbitals.
Question 8.
Why Zn+2 is diamagnetic whereas Cr+3 is paramagnetic ?
Answer:
Zn+2 has electronic configuration [Ar] 4s0 0 3d10
Cr+3 has electronic configuration Ar}] 4s0 0 3d3
Zn+2 contains no unpaired electrons. So it is diamagnetic.
Cr+3 contained three unpaired electrons. So it is paramagnetic.
Question 9.
What is biodegradable polymer ? Give one example.
Answer:
Biodegradable polymers :
The polymers degradable by enzymatic hydrolysis and to some extent by oxidation are called biodegradable polymers. E.g. : Nylon – 2, Nylon – 6, PHBV, Polyglycolic acid, Polylactic acid etc.
Question 10.
Write the names of the monomers of the following poly mers.
a) Bakelite
b) Nylon – 6, 6
Answer:
a) Bakelite :
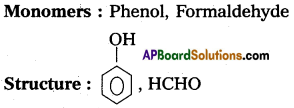
b) Nylon – 6, 6 : The repeating monomeric units of Nylon 6, 6 are hexamethylene diamine and Adipic acid.
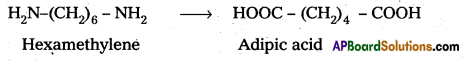
Section – B
Note : Answer any six questions.
Question 11.
What is doping? Explain n-type and p-type semi-conductors.
Answer:
Doping : Conductivity of semi-conductors is too low to be of pratical use. Their conductivity is increased by adding an appropriate amount of suitable impurity. This process is called “doping”.
Doping can be done with an impurity which is electron rich or electron deficient. Extrinsic semi-conductors are of two types.
a) n-type semi-conductors :
It is obtained by adding trace amount of V group element (P, As, Sb) to pure Si or Ge by doping. When P, As, Sb (or) Bi is added to Si or Ge some of the Si or Ge in the crystal are replaced by P or As atoms and four out of five electrons of P or As atom will be used for bonding with Si or Ge atoms while the fifth electron serve to conduct electricity.
b) p-type semi-conductors r It is obtained by doping with impurity atoms containing less electrons i.e., Ill group elements (B, AZ, Ga or In).
When B or Al. is added to pure Si or Ge some of the Si or Ge in the crystal are replaced by B or Al atoms and four out of three electrons of B or Al atom will be used for bonding with “Si” or Ge atoms while the fourth valence electron is missing is called electron hole (or) electron vacancy. This vacancy on an atom in the structure migrates from one atom to another. Hence it facilitates the electrical conductivity.
![]()
Question 12.
Explain the purification of Sulphide ore by froth floatation method.
Answer:
Froth floatation method :
- This method is used to concentrate sulphide ores.
- In this process a suspension of the powdered ore is made with water.
- A rotating paddle is used to agitate the suspension and air is blown into the suspension in presence of an oil.
- Froth is formed as a result of blown of air, which carries the mineral particles.
- To the above slurry froth collectors and stabilizers are added.
- Collectors like pine oil enhance non-wettability of the mineral particles.
- Froth stabilizers like cresol stabilize the froth.
- The mineral particles wet by oil and gangue particles wet by water.
- The froth is light and is skimmed off. The ore particles are then obtained from the froth. By using depressants in froth floatation process, it is possible to separate a mixture of two sulphide ores.
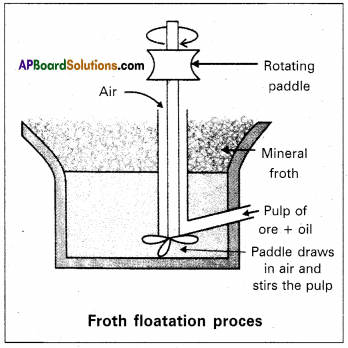
Eg : In the ore containing ZnS and PbS, the depresant used is NaCN. It prevents ZnS from coming to the froth but allows PbS to come with the froth.
Question 13.
State Raoult’s law. The vapour pressure of pure Benzene at a certain temperature is 0.850 bar. A non-volatile, non¬electrolyte solid weighing 0.5 g when added to 39.0 g of benzene (molar mass 78 g mol-1). Vapour pressure of the solution, then is 0.845 bar. What is the molar mass of the solid substance ?
Answer:
Raoult’s law:
The relative lowering of vapour pressure of dilute solution containing non-volatile solute is equal to the mole fraction of solute.
\(\frac{\mathrm{p}_{\mathrm{o}}-\mathrm{p}_{\mathrm{s}}}{\mathrm{p}_0}=\frac{\mathrm{w}_2}{\mathrm{M}_2} \times \frac{\mathrm{M}_1}{\mathrm{w}_1}\)
The various quantities known to us are as follows.
Pi0 = 0.850 bar
P = 0.845bar
M1 = 78 g mol-1
w2 = 0.5 g
w1 = 39 g
Substituting these values in equation
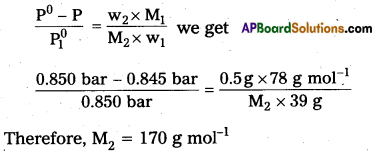
Question 14.
Describe the purification of collidal solutions by Dialysis method with a neat diagram.
Answer:
Colloidal solutions generally contain excess amount of electrolytes and some other soluble impurities. It is necessary to reduce the concentration of soluble impurities to a requisite minimum. The impurities presence required in traces. “The process used for reducing the amount of impurities to a requisite minimum is known as purification of colloidal solution”.
Purification of colloidal solution by Dialysis :
Dialysis :
The process of removing a dissolved substance from a colloidal solution using a suitable membrane is called dialysis.
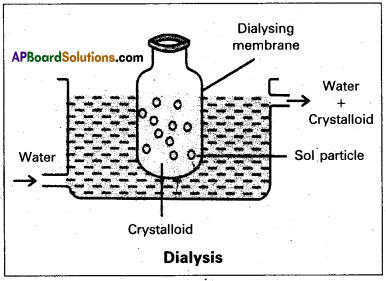
- In a true solution particles can pass through animal membrane (or) cellophane sheet (or) parchment paper but not colloidal particles.
- The apparatus used for the dialysis is called dialyser.
- A bag of suitable membrane containing the colloidal solution is suspended in a vessel containing a continuously flowing water.
- The molecules and ions diffuse through the membrane into the water and pure colloidal solution is left behind in the bag.
Question 15.
Write the equations for reactions of chlorine with the following.
a) Cold and dilute NaOH
b) Excess NH3
c) Ca(OH)2
d) Na2S2O3.
Answer:
(a) Chlorine reacts with cold dil NaOH to form Sodium hypochlorite.
Cl2 + 2 NaOH → NaCl + NaOCl + H20
(b) Chlorine reacts with excess of NaOH to form N2 and NH4Cl.
8 NH3 + 3Cl2 → 6 NH4Cl + N2
(c) Chlorine reacts with Ca(OH)2 to form bleaching powder.
Ca(OH)2 + Cl2 → CaOCl2 + H2O
(d) ‘S’ is precipitated by the reaction of Cl2 with Na2S2O3.
Na2S2O3 + H2O + Cl2 → Na2SO4 + S + 2 HCl
![]()
Question 16.
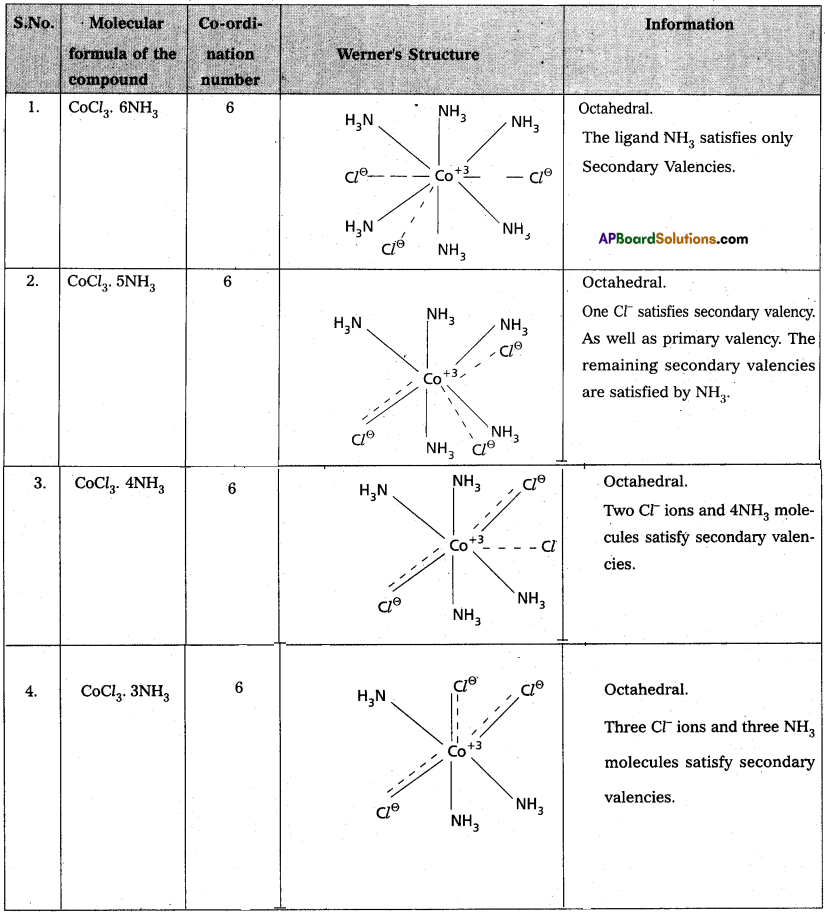
Explain Werner’s theory of Co-ordination compounds.
Answer:
Werner’s theory :
Postulates : 1) Every complex compound has a central metal atom (or) ion.
2) The central metal shows two types of valencies namely primary valency and secondary valency.
A) Primary valency:
The primary valency is numerically equal to the oxidation state of the metal. Species or groups bound by primary valencies undergo complete ionization. These valencies are identical with ionic bonds and are non-directional. These valencies are represented by discontinuous lines (…….) Eg. : CoCl3 contains Co3+ and 3Cl– ions. There are three Primary Valencies or three ionic bonds.
B) Secondary Valency:
Each metal has a characteristic number of Secondary Valencies. They are directed in space around the central metal. The number of Secondary Valencies is called Coordination number (C.N.)(of the metal. These valencies are directional in Nature. For example in CoCl3. 6NH3
Three Cl– ions are held by primary Valencies and 6NH3 mole cules are held by Secondary Valencies. In CuSO4.4NH3 complex SO42- ion is held by two Primary Valencies and 4NH3 molecules are held by Secondary Valencies.
3) Some negative ligands, depending upon the complex, may satisfy both primary and secondaiy valencies. Such ligands, in a complex, which satisfy both primary as well as secondary valencies do not ionize.
4) The primary valency of a metal is known as its outer sphere of attraction or ionizable valency while the Secondary valencies are known as the inner sphere of attraction or coordination sphere. Groups bound by secondary valencies do not undergo ionization in the complex.
Question 17.
What are Hormones? Give one example for each of the following :
a) Steroid hormones.
b) Polypeptide hormones.
c) Amino acid derivatives.
Answer:
Hormones :
Hormone is defined as an “organic compound synthesised by the ductless glands of the body and carried by the blood stream to another part of the body for its function”.
Eg : testosterone, estrogen.
- Example for steroid hormones : Testosterone, Estrogen.
- Example for poly peptide hormones : Insulin.
- Example for Amino acid derivative: Thyroidal hormones thyroxine.
![]()
Question 18.
Explain the mechanism of reaction with one example.
Answer:
Nucleophilic Bimolecular substitution Reaction SN2 :
1) The nucleophilic substitution reaction in which rate depends upon concentration of both reactants is called SN2 reaction.
2) It follows 2nd order kinetics. So it is called bimolecular reaction.
Eg. : Methyl chloride reacts with hydroxide ion and forms methanol and chloride ion.
3) Here the rate of reaction depends upon the concentration of two reactants.
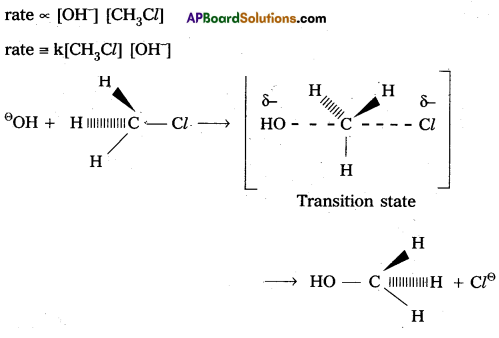
4) In the above mechanism the configuration of carbon atom under attack inverts in much the same way as an umbrella is turned inside out when caught in a strong wind. This process is called inversion of configuration.
5) In transition state the carbon atom is simultaneously bonded to the incoming nucleophile and out going group. It is very unstable.
6) The order of reactivity for SN2 reactions follows :
l°-alkyl halides > 2°-alkyl halides > 3°- alkyl halides.
Section – C
Note : Answer any two of the following questions.
Question 19.
a) What are Galvanic cells ? Explain the working of a Galvanic cells with one example.
Answer:
Galvanic cell : A device which converts chemical energy into electrical energy by the use of spontaneous redox reaction is called Galvanic cell (or) voltaic cell.
Eg : Daniel cell.
Daniel cell: It is a special type of galvanic cell. It contains two half cells in the same vessel.
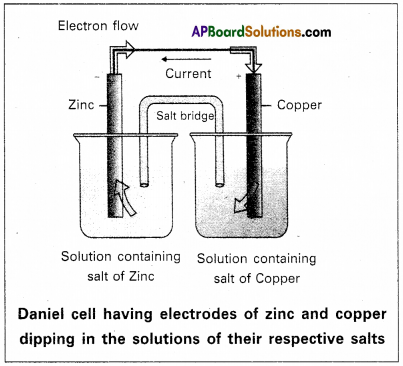
The vessel is devided into two chambers. Left chamber is filled with ZnSO4 (aq) solution and Zn – rod is dipped into it. Right chamber is filled with aq. CuSO4 solution and a copper rod is dipped into it. Process diaphragm acts as Salt bridge. The two half cell’s are connected to external battery.
Cell reactions :
Ion Zn/ZnSO4 half cell, oxidation, reaction occurs
Zn → Zn2+ + 2e–
Ion Cu/CuSO4 half cell, reduction reaction occurs.
Cu+2 + 2e– → Cu
The net cell reaction is
Zn + Cu+2 ⇌ Zn+2 + Cu
Cell is represented as Zn/Zn+2 || Cu2+/Cu
b) Write the differences between order and molecularity of a reaction.
Answer:
Differences between Order and Molecularity of a reaction :
| Order of reaction | Molecularity of reaction |
| i) Order is the sum of powers of concentration terms of reactants in the rate law. aA + bB cC + dD Rate = K [A]x [B]y x + y is the total order of the reaction. x and y may or may not be equal to a and b respe- ctively. Ex : 2H2O2 → 2H2O + O2 is an example for first order reaction. | i) Molecularity is the no. of reacting species taking part in an elementary reaction. Examples :
i) NH4NO2 → N2 + 2H2O (Unimolecular) ii) 2HI → H2+ I2 (Bimolecular) iii) 2NO + O2 → 2NO2 (Trimolecular) |
| ii) It is determined by experiment. | ii) It is determined by reaction mechanism. |
| iii) It may be zero, positive, fra – ctional 0, 1; 2, 1.5 etc., | iii) It can’t be zero, fractional or negative. It is always a whole number. |
| iv) It is applicable to complex and elementary reactions. | iv) It is only applicable to elemen – tary reactions. |
Question 20.
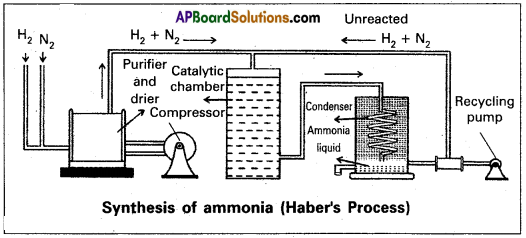
a) How is ammonia manufactured by Haber’s process?
Explain.
Answer:
In Haber process ammonia is directly synthesised from elements (nitrogen and hydrogen).
The principle involved in this is
N2 (g) + 3H2 (g) ⇌ 2NH3 + 92.4 kj
This is a reversible exothermic reaction.
According to Le Chatelier’s principle favourable conditions for the better yield of ammonia are low temperature and high pressure. But the optimum conditions are :
Temperature : 720k
Pressure : 200 atmospheres
Catalyst : Finely divided iron in the presence of molybdenum (Promoter).
Procedure : A mixture of nitrogen and hydrogen in the volume ratio 1:3 is heated to 725 – 775K at a pressure of 200 atmospheres is passed over hot finely divided iron mixed with small amount of molybdenum as promotor. The gases coming out of the catalyst chamber consists of 10 – 20% ammonia gas are cooled and compressed, so that ammonia gas is liquified, and the uncondensed gases are sent for recirculation.
b) How does ozone react with the following :
i) PbS
ii) KI
iii) C2H4
iv) NO
Answer:
i) Reaction with PbS : Black lead sulphide oxidised to white lead sulphate in presence of ozone.
PbS + 4O3 → PbSO4 + 4O2.
ii) Reaction with KI: Moist KI is oxidised to Iodine in presence of ozone.
2KI + H2O + O3 → 2KOH + I2 + O2
iii) Reaction with C2H4 : Ethylene reacts with ozone to form Ethylene ozonoid followed by the hydrolysis to form formaldehyde.
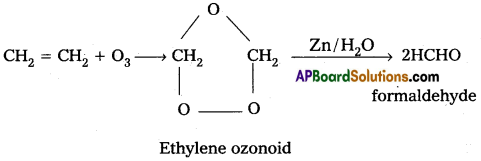
iv) Reactions with NO : Ozone reacts with no to form NO2.
O3 NO → NO2 + O2
Question 21.
Explain the following reactions with suitable example :
i) Riemer – Tiemann reaction.
ii) Cannizaro reaction.
iii) Aldol condensation.
iv) Sandmeyer reaction.
Answer:
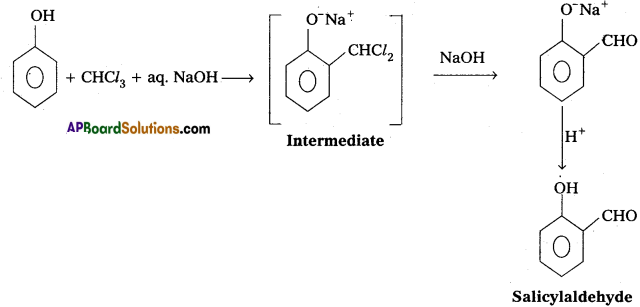
i) Reimer-Tiemann reaction :
Phenol reacts with chloroform in presence of NaOH to form salicylaldehyde (0-Hydroxy benzaldehyde). This reaction is known as Reimer-Tiemann reaction.
ii) Cannizaro reaction :
On treating with concentrated alkali,
aldehydes which do not have any α – hydrogen atom, undergo self oxidation and reduction (disproportionation) reaction. This reaction is called cannizaro reaction.
→ As a result, one molecule of aldehyde is reduced to alcohol while another is oxidised to carboxylic acid salt.
For example :

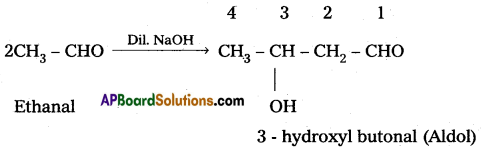
iii) Aldol condensation : When an aldehyde ((or) ketone) having at least one a-hydrogen atom undergo a reaction in the presence of dilute alkali as catalyst to form aldol (or) β – hydroxy aldehydes ((or) ketals in case of ketones), the reaction is called aldol condensation. For example :
Sandmeyer reaction :
Formation of chloro benzene, Brono benzene (or) cyano benzene from benzene diazonium salts with reagents Cu2Cl2/HCl, Cu2Br2/HBr, CuCN/KCN is called sandmayers reaction.