Students can go through AP Inter 1st Year Chemistry Notes 4th Lesson States of Matter: Gases and Liquids will help students in revising the entire concepts quickly.
AP Inter 1st Year Chemistry Notes 4th Lesson States of Matter: Gases and Liquids
→ Graham’s law of diffusion : At a given temperature and pressure the rate of diffusion of a gas is inversely proportional to the square root of density, (or) vapour density or molecular weight.
→ The spontaneous intermixing of two or more gases to form a homogeneous mixture irrespective of the gravitational forces is diffusion.
→ When a gas is allowed to escape from its container through a small hole into vacuum is called effusion.
→ Dalton’s law : “The total pressure exerted by a mixture of perfect gas is the sum of the individual pressures that each gas would exert if it were present alone in the con-tainer at the same temperature”.
Pmix = P1 + P2 + P3 + ………..
This law valid only for mixtures which are chemically non-reacting perfect gases.
![]()
→ Boyle’s law : At constant temperature the volume of a given mass of gas is inversely proportional to its pressure.
→ Charles’ law: At constant pressure the volume of a given mass of gas is directly proportional to the absolute temperature.
→ Avogadro’s law : Equal volumes of all gases under the same conditions of temperature and pressure contain equal number of molecules.
→ Standard temperature or normal temperature means 0°C or 273 K and standard pressure or normal pressure means 1 atmosphere or 76 cm of Hg.
→ Ideal gas equation is PV = nRT.
→ Gases which obey all gas laws at all temperatures and pressures are called ideal gases.
→ Gases which do not obey all gas laws at all temperatures and pressures are real gases.
→ Real gases behave like ideal gases at low pressures and high temperatures.
→ Universal molar gas constant per molecule k = \(\frac{\mathrm{R}}{\mathrm{N}}\) is called Boltzmann constant.
→ RMS speed (urms) is the square root of mean of squares of velocities of all the molecules present in the gas. It can be calculated using urms = \(\sqrt{\frac{3 R T}{M}}\)
→ Average speed (Uav): It is the average speed of all the molecules present in the gas. It can be calculated by using Uav = \(\sqrt{\frac{8 R T}{\pi M}}\)
→ Most Probable speed (Ump): It is the speed possessed by the maximum number of molecules present in the gas. Ump = \(\sqrt{\frac{2 R T}{M}}\)
![]()
→ ump : uav : urms is 1.000 : 1.128 : 1.224.
→ Kinetic energy of’n’ moles of gas = nEk = \(\frac{3}{2}\) nRT.
→ Inter molecular forces are ion-dipole forces, dipole – dipole forces, London dispersion forces & dipole – induced dipole forces.
→ Ion – dipole forces are mainly important in aq. solution of ionic substances.
→ Dipole – dipole forces are due to the electrical interactions among dipoles on neighbouring molecules.
→ The temperature below which the gas can be liquefied by the application of pressure alone is called critical temperature (Tc). The pressure required to liquefy a gas at this temperature is called critical pressure (Pc).
→ The volume occupied by one mole of the substance at the critical temp, and pressure is called critical volume (Vc).
→ Compression factor (Z) is the ratio of the actual molar volume of a gas to the molar volume of a perfect gas.
Z = \(\frac{\text { Pvm }}{\text { RT }}\)
For a perfect gas, Z = 1 and for real gases ‘Z’ varies with pressure.
→ 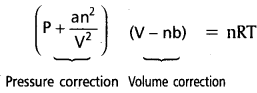 is Vander Waals equation of state, a, b are Vander Waals parameters.
is Vander Waals equation of state, a, b are Vander Waals parameters.
→ Cooling of gas by expansion from high pressure region to low pressure region is called Joule Thomson effect.
→ Vapour pressure : It is pressure exerted by vapour molecules when there is an equilibrium be¬tween liquid phase and vapour phase.
→ Surface tension is defined as the force acting along the surface of a liquid at right angles to anytime of 1 unit length.
→ Viscosity is a measure of the resistance to the flow of liquid. “η is co – efficient of viscosity”.
