Utilizing AP Inter 1st Year Chemistry Model Papers Set 2 helps students overcome exam anxiety by fostering familiarity.
AP Inter 1st Year Chemistry Model Paper Set 2 with Solutions
Time: 3 Hours
Maximum Marks: 60
Note : Read the following instructions carefully.
- Answer all questions of Section – A. Answer ANY SIX questions in Section – B and ANY TWO questions in Section – C.
- In Section – A, questions from Sr. Nos. 1 to 10 are of Very short answer type. Each question carries TWO marks. Every answer may be limited to 2 or 3 sentences. Answer all these questions at one place in the same order.
- In Section – B, questions from Sr. Nos. 11 to 18 are of Short answer type. Each question carries FOUR marks. Every answer may be limited to 75 words.
- In Section – C, questions from Sr. Nos. 19 to 21 are of Long answer type. Each question carries EIGHT marks. Every answer may be limited to 300 words.
- Draw labelled diagrams, wherever necessary for questions in Section – B and Section – C.
Section – A
Note : Answer ALL questions.
Question 1.
Define receptor, sink.
Question 2.
Which oxides cause the acid rain? What is the pH value of acid rain?
Question 3.
Calculate kinetic energy of three moles of CO2 at 27°C (in calories only)
Question 4.
What is disproportionation reaction? Give one example.
![]()
Question 5.
Calculate pH of a 1.0 × 10 8 M solution of HCl.
Question 6.
Lithium salts are mostly hydrated. Why? Give one example.
Question 7.
Why is gypsum added to cement?
Question 8.
What is synthesis gas? How is it prepared?
Question 9.
Define catenation. Write two allotropic crystalline forms of carbon.
Question 10.
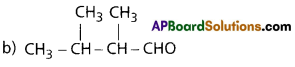
Write IUPAC names of the following compounds :
a) (CH3)3C CH2 C (CH3)3
Section – B
Note : Answer ANY SIX questions.
Question 11.
Write the postulates of kinetic molecular theory of gases.
Question 12.
Define normality. Calculate the normality of oxalic acid solution containing 6.3 g of H2C2O4.2H2O in 500 ml of solution.
Question 13.
Define heat capacity. Derive Cp – Cv = R.
Question 14.
State Lechatelier’s principle. Explain the application of Le Chatelier’s principle on the synthesis of sulphur trioxide (write the effects of temperature and pressure only).
![]()
Question 15.
Which salts are responsible for the hardness of water ? How the hardness of water is removed by Calgon method?
Question 16.
Explain the structure of diborane.
Question 17.
How is acetylene prepared from the following compounds :
a) Calcium carbide
b) 1, 2-dibromoethane
Question 18.
How does ethylene react with the following :
(a) Cl2
(b) HBr
(c) H2SO4
(d) O3
Section – C
Note : Answer ANY TWO of the following questions.
Question 19.
Explain the Quantum numbers and their significances briefly.
Question 20.
Write an essay on s, p, d and f block elements.
Question 21.
Define hybridization. Explain the types of hybridization involving s and p orbitals with one example each.