Students get through AP Inter 1st Year Chemistry Important Questions 4th Lesson States of Matter: Gases and Liquids which are most likely to be asked in the exam.
AP Inter 1st Year Chemistry Important Questions 4th Lesson States of Matter: Gases and Liquids
Very Short Answer Questions
Question 1.
Name the different inter molecular forces experienced hv the molecules of a gas.
Answer:
Various Inter molecular forces:
- Ion-dipole forces
- dipole-dipole forces
- dipole-induced forces
- London dispersion forces.
Question 2.
State Boyle’s law. Give its mathematical expression.
Answer:
Boyle’s law :
“At constant temperature, the volume of a given mass of gas is inversely proportional to the pressure of the gas”.
Thus, V ∝ \(\frac{1}{P}\) ⇔ PV = K (constant)
Question 3.
State Charles’ law. Give its mathematical expression.
Answer:
Charles law :
“At constant pressure, the volume of a given mass of gas is directly proportional to its absolute temperature”.
Thus, V ∝ T ⇔ \(\frac{V}{T}\) = K(constant)
Question 4.
What are isotherms? [Imp.Q]
Answer:
Isotherms :
The curves obtained by plotting volume(V) verses pressure (P) of a gas, at constant temperature, are called isotherms.
Question 5.
What is Absolute Temperature? [Imp.Q]
Answer:
The temperature expressed in kelvin scale is called absolute temperature.
T = 273.15 + t°C and T0 = 273.15 K at 0°C
![]()
Question 6.
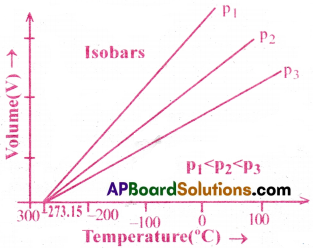
What are Isobars? [Imp.Q]
Answer:
Isobars :
The lines obtained by plotting temperature (T) verses volume(V) at constant pressure, are called isobars.
Question 7.
What is Absolute Zero? [Imp.Q]
Answer:
The lowest hypothetical temperature, at which gases are supposed to occupy zero volume, is called absolute zero.
Question 8.
State Avogadro’s law.
Answer:
Equal volumes of all gases, under the same conditions of temperature and pressure contain equal number of moles.
Mathematically, V ∝ n ⇒ V = kn
Question 9.
What are Isochores?
Answer:
Isochores :
The lines obtained by plotting temperature(T) verses pressure(P) at constant volume, are called isochores.
Question 10.
What are STP conditions? [Imp.Q]
Answer:
STP conditions :
- Standard temperature = 273.15K = 0°C
- Standard pressure = 1 bar.
Question 11.
What is Gram molar volume? [Imp.Q]
Answer:
The volume occupied by one mole of any gas at STP conditions is called Gram molar volume (GMV).
At STP, one mole of any gas occupies 22.4 lit. of volume.
![]()
Question 12.
What is an ideal gas? [TS 22]
Answer:
Any gas which obeys all gas laws, at all temperatures and pressures, is known as ideal gas.
Question 13.
Why the gas constant ‘R’ is called universal gas constant?
Answer:
The value of gas constant ‘R’ is same for all gases. Hence it is called universal gas constant.
Question 14.
Why Ideal gas equation is called Equation of State?
Answer:
Ideal gas equation is a relation between four variables (p, v, n, T) and it describes the state of any gas. Hence it is called equation of state.
Question 15.
Give the values of gas constant in different units. [Imp.Q]
Answer:
Different units of Gas constant ‘R’ :
R = 0.0821 L. atm. K-1 mol-1
= 8.314 × 107 ergs. K-1 mol-1
= 8.314 J. K-1 mol-1
= 1.987 cal. K-1 mol-1
Question 16.
How are the density and molar mass of a gas related?
Answer:
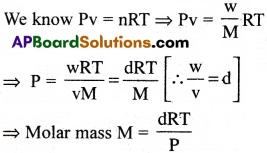
Here, d=Density, P = Pressure of gas;
R = Universal gas constant;
T = Temperature of gas in kelvins scale.
![]()
Question 17.
State Graham’s law of diffusion. [TS 18]
Answer:
Graham’s Law :
“At constant temperature and pressure, the rate of diffusion of a gas is inversely proportional to the square root of its density”.
![]()
Question 18.
Which of the gas diffuses faster among N2, O2, and CH4? Why? [TS 15, 16, 22]
Answer:
The molecular weights of the given gases are N2(28), O2(32) and CH4(16).
From Graham’s law, the lighter gases diffuse faster than heavier gases.
Hence, CH4 diffuses faster.
Question 19.
How many times methane diffuses faster than sulphur dioxide? [TS 19][AP 22]
Answer:
According to Graham’s law of diffusion,
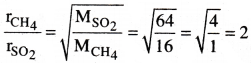
Hence, Methane gas diffuses 2 times faster than SO2.
Question 20.
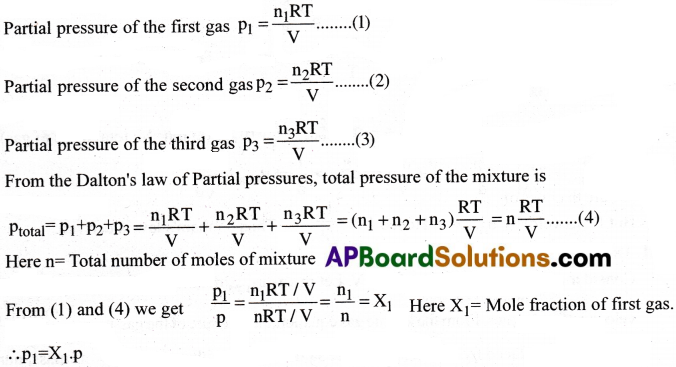
State Dalton’s law of partial pressures. [Imp.Q][IPE’14]
Answer:
Dalton’s law of partial pressures:
“At constant temperature, the total pressure exerted by a mixture of non-reacting gases is equal to the sum of the partial pressures of all the component gases”.
Ptotal = P1 + P2 + …….
Question 21.
Give the relationship between partial pressure of a gas and its mole fraction.
Answer:
Partial pressure of a gas = Mole fraction × Total pressure of that gas
Question 22.
What is aqueous tension? [Imp.Q]
Answer:
The pressure exerted by the saturated water vapour is called aqueous tension.
(or)
The pressure exerted by the water vapour, which is equilibrium with liquid water, is called aqueous tension.
![]()
Question 23.
Give two assumptions of Kinetic molecular theory of gases that do not hold good in explaining the deviation of real gases from ideal behaviour.
Answer:
The two assumptions of KMT which do not explain the deviations of real gases:
- There is no force of attraction between the molecules of a gas.
- Volume of the gas molecules is negligible when compared to the space occupied by the gas.
Question 24.
Give the kinetic gas equation and write the terms In it.
Answer:
Kinetic gas equation is PV = \(\frac{1}{2}\) mnU²rms
P = Pressure of the gas;
V = Volume of the gas;
m = Mass of 1 molecule of the gas
Urms = RMS speed of the gas molecules.
n = Number of moles.
Question 25.
Give an equation to calculate the kinetic energy of gas molecules.
Answer:
Kinetic energy for ‘n’ moles of gas is
K.E = \(\frac{3}{2}\)nRT
R = Universal gas constant
T = absolute temperature
Question 26.
What is Boltzman’s Constant?Give its value. [Imp.Q]
Answer:
The gas constant per molecule is called
Boltzman’s constant.
Boltzman’s constant, K = \(\frac{R}{N}\)
= 1.38 × 10-16 erg/K.molecule
= 1.38 × 10-23 J/K.molecule
Question 27.
What is RMS speed? [Imp.Q]
Answer:
The square root of mean of the squares of the speeds of all molecules of a gas is known as RMS speed (Urms).
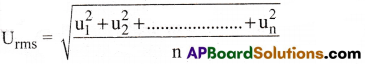
Question 28.
What is Average speed? [Imp.Q]
Answer:
The arithmetic mean of speeds of gas molecules is known as average speed (Uav).
![]()
Question 29.
What is most probable speed? [Imp.Q]
Answer:
The speed possessed by the maximum number of molecules present in the gas is called most probable speed.
Ump = \(\sqrt{\frac{2RT}{M}}\)
![]()
Question 30.
What is the effect of temperature on the speeds of the gas molecules? [Imp.Q]
Answer:
Temperature and speed of gases are directly related.
∴ By the rise of temperature, the speeds of gas molecules increase.
Question 31.
What is the effect of temperature on the kinetic energy of the gas molecules? [Imp.Q]
Answer:
According to Kinetic molecular theory of gases average kinetic energy of gas molecules is directly proportional to absolute temperature, i.e., K.E ∝ Tabs
Question 32.
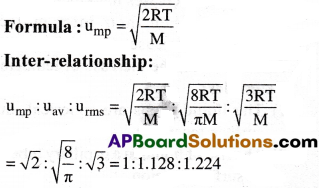
Give the ratio of RMS, average and most probable speeds of gas molecules. [Imp.Q]
Answer:

Question 33.
Why RMS speed is used in the derivation of kinetic gas equation? [Imp.Q]
Answer:
Velocity is a vector quantity. The molecules in a gas will move in all possible directions. In one direction, if the velocity is taken as positive, then in the opposite direction it becomes negative.
Hence, during collisions the resultant velocity of molecules may become zero. To avoid this, all the velocities are squared and the square root of their mean is taken.
Question 34.
What is Compressibility factor? [Imp.Q]
Answer:
The ratio of the actual molar volume of a gas to the molar volume of a perfect gas under the same conditions is called compressibility factor.
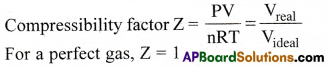
Question 35.
What is Boyle Temperature? [Imp.Q]
Answer:
The temperature at which a real gas exhibits ideal behaviour over a given wide range of pressures is called Boyle’s temperature.
Question 36.
What is critical temperature? Give its value for CO2. [Imp.Q]
Answer:
Critical temperature(Tc) :
The temperature above which, a gas cannot be liquefied by the application at very high pressure is called critical temperature (Tc).
Critical temperature of CO2 gas is 30.98°C.
![]()
Question 37.
What is critical Volume?
Answer:
The volume occupied by one mole of gas at critical temperature and critical pressure, is known as critical volume(Vc).
Question 38.
What is critical Pressure?
Answer:
The pressure required to liquefy a gas at critical temperature is known as critical pressure(Pc).
Question 39.
What are critical constants?
Answer:
The temperature, pressure, volume corresponding to critical point of gas are known as critical temperature(Tc), critical volume (Vc) and critical pressure(Pc). These are known as critical constants.
Question 40.
Define vapour pressure of a liquid. [Imp.Q]
Answer:
The temperature at which the pressure exerted by the vapour of a substance, at a given temperature when it is in equilibrium with its liquid is known as vapour pressure of a liquid.
Question 41.
What are normal and standard boiling points? Give their values for H2O. [Imp.Q]
Answer:
The boiling temperature of a liquid at 1 atm pressure, is called normal boiling point.
The boiling temperature of a liquid at 1 bar pressure, is called standard boiling point.
For water, normal boiling point is 100°C.
For water, standard boiling point is 99.6°C.
Question 42.
Why pressure cooker is used for cooking food on hills? [Imp.Q]
Answer:
At high altitudes, atmospheric pressure is low. So at hills, water boils at lower temperatures when compared to that at sea level. Hence the pressure cooker is used for cooking food, so that boiling point of water can be increased by increasing the pressure above the atmospheric pressure.
Question 43.
What is Surface tension? [AP 18]
Answer:
The force acting per unit length, perpendicular to any line drawn on the surface of a liquid is called surface tension.
SI units: N m-1
Question 44.
What is laminar flow of a liquid?
Answer:
A regular gradation of velocity, in passing from one layer to the next, is called Laminar flow.
![]()
Question 45.
What is coefficient of Viscosity? Give its units.
Answer:
Coefficient of viscosity may be defined as the force of friction required to maintain unit velocity gradient between two parallel layers of unit area of contact.
It is denoted by η.
F = ηA\(\frac{du}{dx}\)
S.I units: N.Sm-2 = Pa.S
CGS units: poise
Short Answer Questions
Question 1.
State and explain Boyle’s law.
Answer:
Boyle’s law :
“At constant temperature, the volume of a given mass of gas is inversely proportional to its pressure”.
Thus, V ∝ \(\frac{1}{P}\), (At constant T,n)
⇒ V = \(\frac{k}{P}\) ⇒ PV = k ………….. (i)
Hence, we conclude that at constant temperature, products of pressure and volume of a fixed amount of a gas is a constant.
From (i), we have
P1V1 = constant and P2V2 = constant
Hence P1V1 = P2V2
Here,
P1 = Initial pressure, V1 = Initial volume
P2 = Final pressure, V2 = Final volume
The curves obtained by plotting volume(V) verses pressure (P) of a gas, at constant temperature, are called isotherms. The shape of Isotherm is ‘Rectangular Hyperbola’.
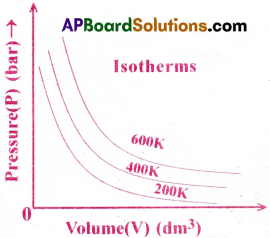
Question 2.
State and explain Charle’s law.
Answer:
Charles law :
“At constant pressure, the volume of a given mass of gas is directly proportional to its absolute temperature”.
Thus,V ∝ T (At constant n,P)
⇒ V = kt (or) \(\frac{V}{T}\) = k ……… (i)
If V1 is the volume of a given mass of a gas at a temperature T1 and V2 is the volume of same mass of gas at temperature T2, then according to Charle’s law,
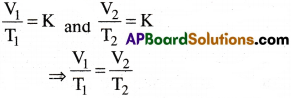
Charles-Gay bussac law :
“At constant pressure, the volume of a given mass of a gas at 0°C increases or decreases by 1/273 part of its original volume at 0°C, for every one degree rise or fall in temperature”. At 0°C, volume of a gas = V0
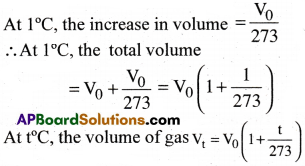
The lines obtained by plotting, temperature versus volume, at constant pressure are called isobars.
Question 3.
Derive Ideal gas equation. [TS 16, 18, 19, 19]
Answer:
The ideal gas equation is derived from Boyles’ law, Charles law, Avogadro’s law.
Let V = Volume, P = Pressure,
T = Absolute temperature and
n = No.of moles of an ideal gas
Boyle’s law :
“At constant temperature, the volume of a given mass of gas is inversely proportional to its pressure”.
Thus, V ∝ \(\frac{1}{P}\) (At constant T,n) ……. (i)
Charles law :
“At constant pressure, the volume of a given mass of gas is directly proportional to its absolute temperature”.
Thus, V ∝ T (At constant n,P) ………… (ii)
Avogadro’s law :
“At constant temperature and pressure, the volume of a gas is directly proportional to the no. of moles”.
Thus, V ∝ n (At constant P, T) ……….. (iii)
Combining the 3 equations(i), (ii), (iii),
we get, V ∝ \(\frac{1}{P}\) × T × n
⇒ V = R\(\frac{1}{P}\)T.n
⇒ PV = nRT
The above equation is called ‘ideal gas equation’ (or) ‘equation of state’.
In the ideal gas equation, ‘R’ is called gas constant and it is independent of the amount of gas.
Value of gas constant R:
The value of gas constant ‘R’ is same for all gases. So, it is called as ‘Universal Gas constant’ .
The value of gas constant depends on units of pressure and volume.
Ex: R = 0.0821 atm.lit.mole-1.K-1.
= 8.314 × 107 ergs. K-1 mol-1
![]()
Question 4.
State and explain Graham’s law of diffusion. [AP 17]
Answer:
Graham’s law of diffusion :
“At constant temperature and pressure the ‘rate of diffusion’ (r) of a given mass of gas is inversely proportional to the square root of its density (d). r ∝ \(\frac{1}{\sqrt{d}}\)
If r1, r2 are rates of diffusions & d1, d2 are densities of two gases at constant temperature & pressure then from Graham’s law,

We know that molar mass(M) of a gas is directly proportional its densities(d)

Here M1, M2 are molar masses of 2 gases.
We also know the molar mass of a gas is directly proportional to its vapour density

Question 5.
State Dalton’s law and derive dalton’s law from kinetic gas equation. [AP 16]
Answer:
Dalton’s law of partial pressure:
“At constant temperature, the total pressure exerted by a mixture of non-reacting gases is equal to the sum of the partial pressures of all the components gases”.
∴ Total pressure, ptotal = p1 + p2 ……… + pn.
Derivation:
Consider a gas in a vessel of volume V. Let m1,n1,u1rms denote the mass, no.of molecules and RMS velocity of molecules. From the kinetic gas equation, the pressure
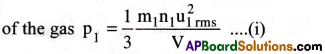
If the gas is replaced by another gas in the same vessel, with m2, n2, u2rms as mass, number of molecules and RMS velocity of molecules, then its pressure

Suppose the two gases are taken in the same vessel of volume V and if P is the total pressure of the mixture then

∴ ptotal = p1 + p2 (from (i) & (ii)
Thus Dalton’s law of partial pressure is derived.
Question 6.
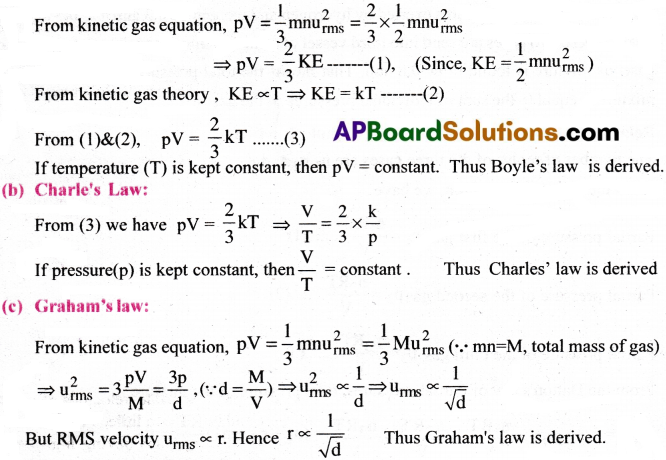
Deduce (a) Boyle’s law (b) Charle’s law from kinetic gas equation. [TS 15, 20, 22][AP 16, 19]
Answer:
(a) Boyle’s law:
From kinetic gas equation
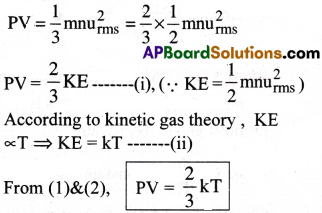
If temperature (T) is kept constant, then
PV = constant
Thus, Boyle’s law is derived
(b) Charle’s Law : [AP 22]
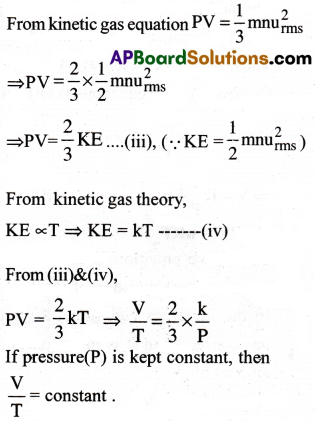
Thus, Charles’ law is derived.
Question 7.
Deduce (a) Graham’s law and (b) Dalton’s law of partial pressures from kinetic gas equation. [AP 19,20]
Answer:
Graham’s law:
“At constant temperature and pressure the rate of diffusion (r)of a gas is inversely proportional to the square root of its density (d)”. [AP, TS 15,16]
From kinetic gas equation
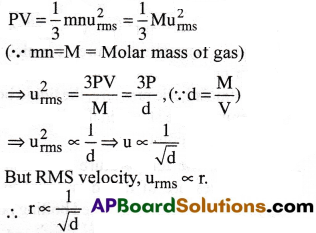
Thus Graham’s law is derived from kinetic gas equation.
Dalton’s law of partial pressures : [AP 22]
“The total pressure exerted by a mixture of non-reacting gases is equal to the sum of the partial pressures of component gases at a given temperature and volume”.
Consider a gas in a vessel of volume V. Let m1, n1, u1rms denote the mass, number and RMS velocity of molecules. From the kinetic gas equation, the pressure of the gas
![]()
If the gas is replaced by another gas in the same vessel, with m2, n2, u2rms as mass, number and RMS velocity of molecules, then
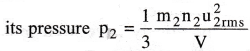
Suppose the two gases are taken in the same vessel and if P is the total pressure of the mixture then

Thus Dalton’s law is derived from kinetic gas equation.
![]()
Question 8.
Derive an expression for kinetic Energy of gas molecules.
Answer:
From Kinetic gas equation, we have
PV = \(\frac{1}{3}\)mnc² ……(1)
For 1 mole of gas, n = N = Avogadro number and mN = M = Molar mass
Then from (1),
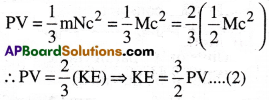
The Ideal gas equation for 1 mole of gas is PV = RT
Hence, from (2), KE = \(\frac{3}{2}\)RT
∴ KE of ‘n’ moles of gas is KE = \(\frac{3}{2}\)nRT
Question 9.
Define (a) rms (b) average and (c) most probable speeds of gas molecules. Give their interrelationship.
Answer:
(a) RMS velocity (urms) :
The square root of mean of squares of individual velocities of gas molecules is called Root Mean Square (RMS) velocity.
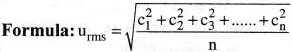
(b) Average velocity (uav) :
The ratio of the sum of the velocities of gas molecules to the total number of molecules is called average velocity.
![]()
(c) Most probable velocity(ump) :
The velocity possessed by maximum number of gas molecules is called most probable velocity.
Question 10.
Explain the physical significance of Vander Waals parameters.
Answer:
Vander Waals equation:
(P + \(\frac{an^2}{V^2}\))(V – nb) = nRT
Here, a, b = Vander Waal’s parameters
P = Pressure of the gas
V =Volume of the container
R = Gas constant
T = Absolute temperature.
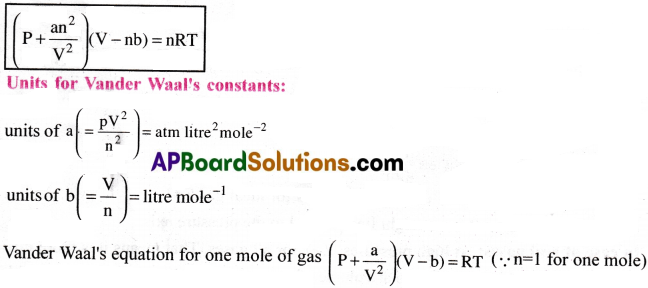
Units of ‘a’ : bar lit² mole-2.
Units of ’b’ : lit. mol-1.
Significance :
The values of the 2 parameters a, b depend upon the nature of the gas.
‘a’ is the measure of magnitude of inter molecular forces (attractive) within the gas and is independent of temperature and pressure. If value of ‘a’ is high, the gas can easily be liquefied.
‘b’ is the effective volume of the gas molecule. It indicates the effective size of the gas molecules. If the value of ‘b’ is constant over a long range of temperatures and pressures then the gas cannot be compressed easily.
Question 11.
What is surface tension of liquids? Explain the effect of temperature on the surface tension of liquids. [AP17]
Answer:
The force acting along the surface of a liquid at right angles to any line of ‘unit length’, is called Surface tension(y).
Units: Nm-1.
The phenomenon of surface tension is due to the existence of strong intermolecular forces of attraction in liquids.
Effect of temperature:
Increase in the temperature increases the kinetic energy of molecules. Then their inter molecular attractions decrease. So surface tension decreases with the increase of temperature.
Examples:
- The liquid drops are spherical, due to surface tension.
- The rise of liquid in a capillary tube is due to surface tension.
Question 12.
What is vapour pressure of liquids? How the vapour pressure of a liquid to related to its boiling point?
Answer:
The pressure exerted by vapour molecules on the surface area of a liquid, when liquid phase and vapour phase are in equilibrium, at a given temperature, is called vapour pressure of the liquid.
When temperature of a liquid is increased, the rate of vapourisation increases. The temperature at which the vapour pressure of a liquid becomes equal to the atmospheric pressure, the liquid boils. The temperature at which the liquid boils is called boiling point.
Thus, the temperature at which the vapour pressure of liquid is equal to the atmospheric pressure is called boiling point. The boiling temperature of a liquid at 1 atm pressure, is called normal boiling point. The boiling temperature of a liquid at 1 bar pressure, is called standard boiling point.
![]()
Question 13.
Define viscosity and coefficient of viscosity. How does the viscosity of liquids varies with temperature.
Answer:
Viscosity :
The property of resistance to flow is called Viscosity.
Viscosity of a liquid is a measure of internal friction of moving fluid.
If viscosity increases then flow of liquid decreases.
Liquids which flow rapidly have low internal resistance. So their viscosity is less. Liquids which flow slowly have high internal resistance. So their viscosity is high.
Coefficient of viscosity : It is defined as the force of friction required to maintain unit velocity gradient between two parallel layers of unit area of contact.
Viscosity of liquids decreases with the increase of temperature. Because at high temperatures, molecules have high kinetic energy and they can overcome the intermolecular forces to ‘slide’ one another between layers.
Long Answer Questions
Question 1.
Write notes on intermolecular forces.
Answer:
Inter molecular Forces:
The forces of attraction and repulsion between the interacting atoms and molecules are called Inter molecular forces.
Types of Inter Molecular forces:
1) Ion-Dipole forces:
These forces are observed between an ion and a dipole (Exrwater molecule) in aqueous solutions.
Ex: NaCl in water.
When ionic compounds like NaCl dissolve in water, they dissociate into their component ions Na+ and Cl–. Now, the water molecules orient in such a way that the +ve end of the dipole is near to the anion and the -ve end of the dipole is near to the cation.
The magnitude of the interaction energy is E = Zµ/r²
where Z = charge on the ion,
µ = dipole moment
r = distance between ion and dipole.
2) Dipole-Dipole forces :
These are the weak attractive forces observed between neutral polar molecules.
Ex: HCl shows dipole-dipole forces. These forces are due to the electrical interactions among dipoles on neighbouring molecules.
These forces are generally weak and are significant only when the molecules are in close contact.
The strength of these forces depends on the sizes of the dipole moments involved. The higher the dipole moment, the greater is the strength of interactions.
Between stationary molecules the interaction energy is proportional to \(\frac{1}{r^3}\) and for rotating molecules it is r proportional to \(\frac{1}{r^6}\)
where ‘r’ is the distance between the polar molecules.
3) Induced dipole-induced dipole forces (London dispersion forces):
The force of attraction between two temporary dipoles is known as London dispersion forces.
Ex: Benzene (C6H6) molecules have these type of forces.
These forces result from the motion of electrons in an atom. The electrons cloud in atoms (or) molecules can be distorted by a nearby electric filed. This property is known as Polarisability.
A smaller molecule or atom is less polarisable and has smaller dispersion forces. A larger molecule is more polarisable and has large dispersion forces. These forces are always attractive.
The magnitude of the interaction energy is proportional to \(\frac{1}{r^6}\), where ’r’ is the distance between the two interaction particles.
4) Dipole – Induced Dipole forces :
These forces operate between polar molecules with permanent dipole moments and the molecules lacking permanent dipole moment.
Permanent dipole of the polar molecule induces dipole on the electrically neutral molecule by deforming the electron cloud. The interaction energy is directly proportional to \(\frac{1}{r^6}\), where ‘r’ is the distance between the molecules.
The magnitude of induced dipole moment also depends on the magnitude of the dipole moment of permanent dipole and polarisability of neutral molecule.
Question 2.
State Boyle’s law, Charles law and Avogadro’s law and derive ideal gas equation.
Answer:
The ideal gas equation is derived from Boyles’ law, Charles law, Avogadro’s law.
Let V = Volume, P = Pressure,
T = Absolute temperature and
n = No.of moles of an ideal gas
Boyle’s law :
“At constant temperature, the volume of a given mass of gas is inversely proportional to its pressure”.
Thus, V ∝ \(\frac{1}{P}\) (At constant T,n) ……. (i)
Charles law :
“At constant pressure, the volume of a given mass of gas is directly proportional to its absolute temperature”.
Thus, V ∝ T (At constant n,P) ………… (ii)
Avogadro’s law :
“At constant temperature and pressure, the volume of a gas is directly proportional to the no. of moles”.
Thus, V ∝ n (At constant P, T) ……….. (iii)
Combining the 3 equations(i), (ii), (iii),
we get, V ∝ \(\frac{1}{P}\) × T × n
⇒ V = R\(\frac{1}{P}\)T.n
⇒ PV = nRT
The above equation is called ‘ideal gas equation’ (or) ‘equation of state’.
In the ideal gas equation, ‘R’ is called gas constant and it is independent of the amount of gas.
Value of gas constant R:
The value of gas constant ‘R’ is same for all gases. So, it is called as ‘Universal Gas constant’ .
The value of gas constant depends on units of pressure and volume.
Ex: R = 0.0821 atm.lit.mole-1.K-1.
= 8.314 × 107 ergs. K-1 mol-1
![]()
Question 3.
Write notes on diffusion of Gases.
Answer:
Diffusion :
The phenomenon of mixing of two or more gases against gravitational forces to form a homogeneous mixture is known as ‘Diffusion’,
Applications:
- For the separation of uranium isotopes by converting into hexafluorides.
- The molecular weight of unknown gas is determined.
- Fous and poisonous gases are diluted by diffusion.
- Ansils’ alaram used in coal mines works on diffusion principle.
Question 4.
State and explain Dalton’s law of partial pressures.
Answer:
Dalton’s law of partial pressure :
“At constant temperature, the total pressure exerted by a mixture of non-reacting gases is equal to the sum of the partial pressures of all the components gases”.
∴ Total pressure, ptotal = p1 + p2 ……….. + pn.
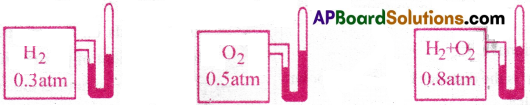
Explanation :
Let three vessels of equal volumes are taken and attached with “manometers”. Let n1 moles of hydrogen and n2 moles of oxygen are taken in the first and second vessels respectively. Let the pressures exerted by hydrogen and oxygen be 0.3 atm and 0.5 atm. respectively. These two gases are send into third vessel of same volume. Then the total pressure of the gaseous mixture is found to be 0.8 atm. That means the total pressure exerted by the gaseous mixture is equal to the sum of individual pressures of the gases.
Relation between partial pressure and mole fraction:
The number of moles of the three gases are n1, n2 and n3.
From the Ideal gas equation, we have
Similarly we get p2 = X2P and p3 = X3P
Conclusion :
Partial pressure of a gas in the gaseous mixture is equal to the product of its mole fraction and total pressure of mixture.
Question 5.
Write the postulates of kinetic molecular theory of gases. [TS 15,17,18,22][AP 16,19]
Answer:
The postulates of kinetic molecular theory of gases:
- Every gas contains large number of tiny particles called molecules.
- The gas molecules move randomly in all directions with high velocities.
- There will be no attractive or repulsive forces among gas molecules.
- There will be no effect of gravitational force on the movement of gas molecules.
- The volume of gas molecules is negligible when compared to the volume of the container.
- Pressure of gas is due to collisions of the gas molecules on the walls of the container.
- All collisions are perfectly elastic.
- The average kinetic energy of a gas is directly proportional to the absolute temperature. Thus, KE ∝ T
Question 6.
Deduce gas laws from the Kinetic gas equation.
Answer:
(a) Boyle’s law :
(d) Dalton’s law of partial pressures:
Consider a gas in a vessel of volume V. Let m1, n1, u1rms denote the mass, number and RMS
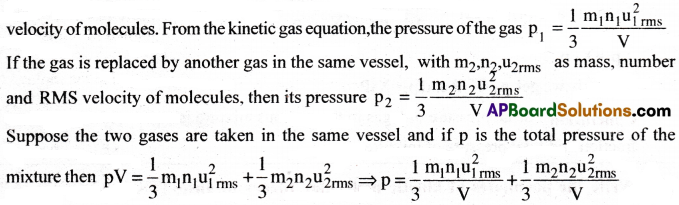
∴ p = p1 + p2. Thus Dalton’s law is derived.
(e) Avogadro’s law :
Consider equal volumes (V) of two different gases at the same temperature and pressure (p).
Then from the kinetic gas equation we have pV = \(\frac{1}{3}\)m1n1u1², pV = \(\frac{1}{3}\) m2n2u2²
⇒ m1n1u1² = m2n2u2² ……….. (1)
Since, the temperatures of the two gases are same, their average K.E’s are equal.
⇒ \(\frac{1}{2}\) m1u1² = \(\frac{1}{2}\) m2u2² ⇒ m1u1² = m2u2² ……….. (2)
From (1) & (2) we get n1 = n2
Therefore at constant temperature and pressure, equal volumes of all gases contain equal number of moles. Thus Avogadro’s law is derived.
![]()
Question 7.
Explain Maxwell-Boltzman distribution curves of molecular speeds and give the important conclusions. Discuss the effect of temperature on the distribution of molecular speeds.
Answer:
According to kinetic theory, the molecules in a gas move randomly in all directions. During this random motion, they collide with each other and also with the walls of the container. As a result, the molecular velocities constantly change from a very low value to a very high value.
The distribution of velocities of molecules is understand from the “Maxwell-Boltzman distribution curves.”
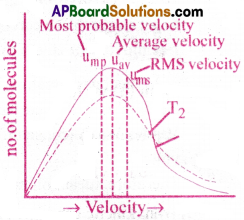
Important Conclusions:
- There are no molecules with zero velocity.
- Very few molecules have the lowest and highest velocities.
- The velocities of the most of the molecules are near to the average value.
- As the velocity of the molecules increases, a fraction of molecules having a particular velocity increases up to a peak value and then decreases.
- The peak of the curve indicates the velocity possessed by most of the gas molecules.
It is called “most probable velocity(Ump)”.
Effect of Temperature :
As the temperature increases, most probable velocities of molecules increase. The distribution curve shifts to the right side and broadens a little. From this, it can be understood that, at high temperatures, the number of the molecules having high velocities increases.
Question 8.
Write notes on the behaviour of real gases and their deviation from ideal behaviour.
Answer:
The deviation of the real gases from the ideal behaviour can be studied in terms of a quantity called Compressibility factor.
Compressibility Faetor (Z):
It is the ratio of the molar volume of a gas to the molar volume of ideal gas, under the same conditions.

For a ideal gas, Z = 1. So for other values of Z, real gases differ from ideal behaviour.
In case of ideal gas:
- For ideal gas, PV = nRT ⇒ \(\frac{PV}{nRT}\) = 1 ⇒ Z = 1.
Therefore at all temperatures and pressures for ideal gas Z = 1. - The graph of Z vs P is a straight line parallel to the pressure axis.
In case of real gases :
At high pressures, Z>1 for all gases. That means it is more difficult to compress. Z>1 means that the molar volume of a gas is greater than that expected for ideal gas. At intermediate pressure, for most of the gases Z<1. They are more compressible than expected from ideal behaviour.
Ex: CH4 and CO2 (At low and moderate pressures.)
At very low pressures all gases have Z ≈ 1 and they behave like ideal gas.
In general, the behaviour of gas becomes more ideal when the pressure is very low.
The temperature at which a real gas obeys ideal gas law over an appreciable range of pressures is called Boyle temperature.
Question 9.
Derive the Vander waal equation of state. Explain the importance of Vander Waal’s gas equation. [AP 15]
Answer:
Vander Waals Equation :
According to Vander Waal, the deviations of a gas from ideal gas equation are due to intermolecular interactions.
He modified the perfect gas equation by introducing two correction terms, one for volume and the other for pressure.
(i) Volume correction :
The repulsive interactions between two molecules cannot allow them to get closer than a certain distance. Hence, for the molecules, the available volume for the free movement is less than the volume of the container V.
∴ In ideal gas equation, a volume correction is made by changing V to (V-nb)
Here, ‘b’ is called correction factor in volume. It is also known as excluded volume or Vander Waal’s constant. Also ‘n’ is number of moles of the gas present in the vessel of volume ‘V’.
Therefore, ideal volume Vi=(V-nb)
(ii) Pressure correction :
The effect of attractive interactions between molecules is to reduce the pressure that the gas exerts. As the attractions slow down the molecules, they strike the waals less frequently and the impact is weak. Thus, there is reduction in pressure. Reduction in pressure is directly proportional to square of the molar concentration (n/v)
Reduction in pressure p ∝ (\(\frac{n}{v}\))² ⇒ p = latex]\frac{an^2}{v^2}[/latex], where ‘a’ is a constant.
This value of ‘a’ measures the force of attraction between the molecules of a gas.
Greater the value of ’a’, greater is the strength of Vander Waal’s interactions.
Vander Waal’s equation after making corrections in volume and pressure, for ‘n’ moles gas is
Importance :
Ideal gas equation applicable to gases only at low pressure and high temperature. But Vander waal’s gas equation applicable to all gases, at all conditions.
![]()
Question 10.
Explain the principle underlying tiie liquefaction of gases.
Answer:
The liquefaction of a gas can be achieved by decreasing its temperature and increasing pressure on it. In order to liquefy a gas it must be cooled below its critical temperature.
Ex : The critical temperature of CO2 is 30.98°C i.e., CO2 remains in gaseous state up to 30.98°C, But at and below 30.98°C & 73 atm. pressure it converts into liquid.
In the process of liquefaction of gases is done on the basis of a special technique based on intermolecular forces.
If we reduce the velocities of gas molecules to lower values, then the neighbouring molecules attract each other, get cooled and condense to a liquid. In order to make this, the gas should be allowed to expand without supplying any heat from outside. In this process the attractions between the neighbouring molecules will be lowered. In doing so, the gas molecules convert some of their kinetic energy into potential energy and travel slowly. As a result, the average velocity decreases and therefore the temperature of the gas decreases and the gas cools down. In order to happen this, the gas should be allowed to expand through a narrow opening called throttle. This way of cooling a gas by expansion, from high pressure to low pressure is called Joule-Thomson effect.
If the process is repeated several times, the gas condenses to a liquid.
Question 11.
Write notes on the following properties of liquids
a) Vapour pressure
b) Surface tension
c) Viscosity
Answer:
a) Vapour Pressuretyhe pressure exerted by vapour molecules on the surface area of a liquid, when liquid phase and vapour phase are in equilibrium, at a given temperature, is called vapour pressure of the liquid.
When temperature of a liquid is increased, the rate of vapourisation increases. The temperature at which the vapour pressure of a liquid becomes equal to the external pressure, the liquid boils. The temperature at which the liquid boils is called boiling point.
Thus, the temperature at which the vapour pressure of liquid is equal to the atmospheric pressure is called boiling point.
The boiling temperature of a liquid at 1 atm pressure, is called normal boiling point.
The boiling temperature of a liquid at 1 bar pressure, is called standard boiling point.
b) Surface Tension :
The force acting along the surface of a liquid at right angles to any line of ‘unit length’, is called Surface tension(γ). Units C.G.S system = dynes cm-1. S.I units: Nm-1. The phenomenon of surface tension is due to the existence of strong intermolecular forces of attraction in liquids.
Effect of temperature :
Increase in the temperature increases the kinetic energy of molecules. Then their inter molecular attractions decrease. So surface tension decreases with the increase of temperature.
Examples:
- The liquid drops are spherical, due to surface tension.
- The rise of liquid in a capillary tube is due to surface tension.
c) Viscosity :
The property of resistance to flow is called Viscosity. Viscosity of a liquid is a measure of its frictional resistance to the flow of liquid.If viscosity increases then flow of liquid decreases.Liquids which flow rapidly have low internal resistance. So their viscosity is less. Liquids which flow slowly have high internal resistance. So their viscosity is high.
Examples:
- Glass is not a solid. It is a super-cooled liquid with a very high viscosity.
- H2SO4 is viscous, due to H-bonding.
Multiple Choice Questions
Question 1.
What will be the minimum pressure required to compress 500dm³ of air at 1 bar to 200 dm³ at 30°C?
1) 2.5 bar
2) 2.9 bar
3) 2.8 bar
4) 2.7 bar
Answer:
1) 2.5 bar
Solution:
Given data: V1 = 500dm³, V2= 200dm³
P1 = 1 bar, P2 = ?
From Boyle’s law, P1V1 = P2V2
![]()
Question 2.
A vessel of 120ml capacity contains a certain amount of gas at 35″C and 1.2 bar pressure. The gas is transferred to another vessel of volume 180ml at 35°C. What would be its pressure?
1) 0.5 bar
2) 0.6 bar
3) 0.7 bar
4) 0.8 bar
Answer:
4) 0.8 bar
Solution:
Given data:V1 = 120 ml, V2 = 180 ml
P1 = 1.2 bar, P2 =?
From Boyle’s law, P1V1 = P2V2
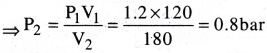
Question 3.
Using the equation pV = nRT, at a given temperature, density of a gas is proportional to
1) pressure
2) temperature
3) no.of moles
4) none
Answer:
1) pressure
Solution:
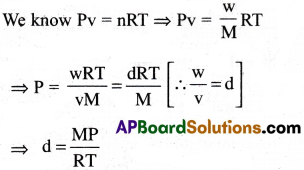
At constant R, T we have d ∝ p
![]()
Question 4.
At 0″C, the density of a certain oxide of a gas at 2 bar is same as that of dinitrogen at 5 bar. What is the molecular mass of the oxide?
1) 72 gmol-1
2) 71 gmol-1
3) 70 gmol-1
4) 75 gmol-1
Answer:
3) 70 gmol-1
Solution:
Given data: P1 = 2 bar, P2 = 5,
M1 = ? M2 = 28(N2)

Question 5.
Pressure of lg of an ideal gas A at 27°C is found to be 2 bar. When 2 g of another ideal gas B is introduced in the same flask at same temperature the pressure becomes 3 bar. Find a relationship between their molecular masses.
1) MB = MA
2) MB = 3MA
3) MB = 2MA
4) MB = 4MA
Answer:
4) MB = 4MA
Solution:
Given data: PA = 2 bar, PB = 1 bar
WA = 1 gm, WB = 2 gm

Question 6.
The drain cleaner, Drainex contains small bits of aluminium which react with caustic soda to produce dihydrogen. What volume of dihydrogen at 20°C and one bar will be released when 0.15g of aluminium reacts?
1) 204.8 mL
2) 202.8 mL
3) 206.8 mL
4) 205.8 mL
Answer:
2) 202.8 mL
Solution:
From the given data, we have
2Al + 2NaOH + 2H2O → 2NaAlO2 + 3H2
Hence, 2 gram atoms of Al liberates 3 moles of H2 at NTP
Thus 2 × 27 gms Al liberates 3 × 22.4L of H2
Then 0.15gms of Al liberates × L
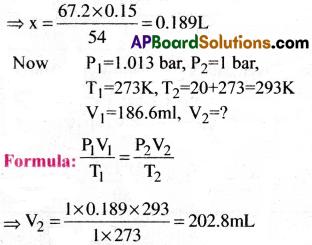
Question 7.
What will be the pressure exerted by a mixture of 3.2 g of methane and 4.4 g of carbon dioxide contained in a 9 dm³ flask at 2TC?
1) 8.414 × 104Pa
2) 8.514 × 104Pa
3) 8.314 × 104Pa
4) 8.614 × 104Pa
Answer:
3) 8.314 × 104Pa
Solution:
No.of moles of CH4, nCH4 = \(\frac{3.2}{16}\) = 0.2
No.of moles of CO2, nCO2 = \(\frac{4.4}{44}\) = 0.1
Total no.of moles
n = nCH4 + nCO2 = 0.2 + 0.1 = 0.3
We know R = 8.314 Pam³k-1mole-1
T = 27 + 273K = 300K V = 9dm³ = 9 × 10-3m³

Question 8.
What will be the pressure of the gaseous mixture when 0.5L of H2 at 0.8 bar and 2.0 L of dioxygen at 0.7 bar are introduced in a 1L vessel at 27°C?
1) 1.9 bar
2) 1.8 bar
3) 1.7 bar
4) 1.6 bar
Answer:
2) 1.8 bar
Solution:
Given data:
H2 gas O2 gas
P1 = 0.8 bar, P2 = 0.7 bar,
V1 = 0.5L V2 = 2.0L
PV = P1V1 = P2V2

Question 9.
Density of a gas is found to be 5.46g/ dm³ at 27°C at 2 bar pressure. What will be its density at STP?
1) 3g/dm³
2) 5g/dm³
3) 4g/dm³
4) 6g/dm³
Answer:
1) 3g/dm³
Solution:
Given data:
P1 = 2 bar, P2 = 1 bar,
T1 = 27°C + 273 = 300K T2 = 273 K
d1 =5.46 d/dm³, d2 = ?
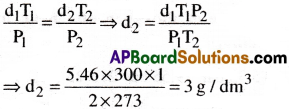
![]()
Question 10.
34.05ml of phosphorus vapour weighs 0.00625g at 546°C and 0.1 bar pressure. What is the molar mass of phosphorus?
1) 125.9g/mol
2) 120.9g/mol
3) 124.9 g/mol
4) 126.9 g/mol
Answer:
3) 124.9 g/mol
Solution:
Given data:
V = 34.05 ml = 34.05 × 10-3 L
P = 0.1 bar
w = 0.0625 gms
R = 0.08314 bar dm³ K-1mol-1
T = 546°C + 273 = 819K
Formula:

Question 11.
A student forgot to add the reaction mixture to the round bottomed flask at 27°C but instead he/she placed the flask on the flame. After a lapse of time, he realized his mistake, and using a pyrometer he found the temperature of the flask was 477°C. What fraction of air would have been expelled out?
1) 0.9
2) 0.6
3) 0.7
4) 0.8
Answer:
2) 0.6
Solution:
Given data: T1 = 27 + 273 = 300K
T2 = 477 + 273 = 750K
Let the volume of flask be V1 = V ml
From Charles law,
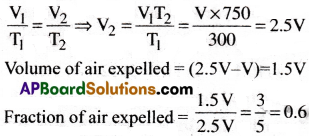
Question 12.
Calculate the temperature of 4.0mol of a gas occupying 5 dm³ at 3.32 bar. (R= 0.083 bar dm³K-1mol-1)
1) 40 K
2) 30 K
3) 50 K
4) 20 K
Answer:
3) 50 K
Solution:
Given data:
P = 3.32 bar V = 5 dm³
n = 4.0mol, R= 0.083 bar dm³K-1mol-1
Formula : PV = nRT
⇒ T = \(\frac{PV}{nR}=\frac{3.32\times5}{4\times0.083}\) = 50K
Question 13.
Calculate the total number of electrons present in 1.4g of dinitrogen gas.
1) 4.216 × 1023
2) 4.416 × 1022
3) 4.311 × 1022
4)4.510 × 1023
Answer:
1) 4.216 × 1023
Solution:
No.of moles of N2 = \(\frac{1.4}{28}\) = 0.05
Avogadro’s number N= 6.023 × 1023
No.of electrons in N2 molecule = 2 × 7 = 14
Total No. of electrons
= No.of moles × Avogadro number × No.of electrons in N2
= 0.05 × 6.023 × 1023 × 14 =4.216 × 1023
Question 14.
How much time would it take to distribute one Avogadro number of wheat grains. If 1010 grains are distributed each second?
1) 1.609 × 106 years
2) 1.809 × 106 years
3) 1.909 × 106 years
4) 1.709 × 106 years
Answer:
3) 1.909 × 106 years
Solution:
Time to distribute 1010 wheat grains = 1 sec.
∴ Time to distribute one Avogadrao number 6.023 × 1023 of wheat grains
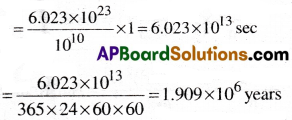
Question 15.
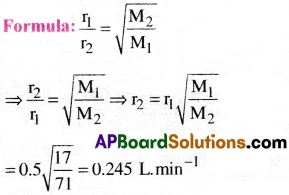
Ammonia gas diffuses through a fine hole at the rate 0.5 L. min-1. Under the same conditions find the rate of diffusion of chlorine gas.
1) 0.245 L. min-1
2) 0.247 L. min-1
3) 0.249 L. min-1
4) 0.246 L. min-1
Answer:
1) 0.245 L. min-1
Solution:
Given data:
Rate of diffusion of NH3 is r1 = 0.5 L.min-1
Molar mass of NH3 is M1 = 17
Molar mass of Cl2 is M2 = 71
Rate of diffusion of Cl2 is r2 =?
Question 16.
Find the relative rates of diffusion of CO2 and Cl2 gases.
1) 1.277 : 1
2) 1.257 : 1
3) 1.267 : 1
4) 1.247 : 1
Answer:
3) 1.267 : 1
Solution:

Question 17.
If 150mL Carbon Monoxide effused in 25 seconds, what volume of methane would effuse in same time.
1) 188.5 ml
2) 198.5 ml
3) 178.5 ml
4) 168.5 ml
Answer:
2) 198.5 ml
Solution:
Given data:
Rate of effusion of CO, r1 = \(\frac{v}{t}=\frac{150}{25}\) ml/sec
Molecular weight of CO, M1 = 28
Rate of effusion of CH4, r2 = \(\frac{v}{t}=\frac{v}{25}\) ml/sec
Molecular weight of CH4, M2 = 16

Question 18.
Hydrogen chloride gas is sent into a 100 meter tube from one end ‘A’ and ammonia gas from the other end ‘B’ under similar conditions. At what distance from ‘A’ will be the two gases meet.
1) 40.48 m
2) 41.46 m
3) 50.48 m
4) 60.48 m
Answer:
1) 40.48 m
Solution:
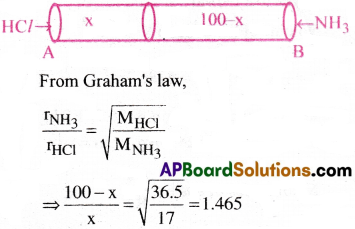
⇒ 100 – x = 1.465x ⇒ 1.465x + x = 100
⇒ 2.465x = 100 ⇒ x =40.48m
∴ x = 40.48 meters from the end ‘A’.
![]()
Question 19.
Calculate the total pressure in a mixture of 8 g of dioxygen and 4 g of dihydrogen confined in a vessel of 1 dm3 at 27°C. R = 0.083 bar dm³ k-1mol-1.
1) 55.025 bar
2) 57.025 bar
3) 56.025 bar
4) 58.025 bar
Answer:
3) 56.025 bar
Solution:
Given data; Volume. V = 1dm³
Temperature, T = 27°C + 273 = 300K.
Gas constant. R = 0.083 bar dm³K-1mol-1
No. of moles of O2, nO2 = \(\frac{8}{32}\) = 0.25;
No. of moles of H2, nH2 = \(\frac{4}{2}\) = 2
Total no. of moles, n = nO2 + nH2
= 0.25 + 2 = 2.25
From the ideal gas equation PV = nRT
![]()
Question 20.
Calculate the total pressure in a mixture of 3.5g of dinitrogen 3.0g of dihydrogen and 8.0g dioxygen confined in vessel of 5dm³ at 27°C. (R = 0.083 bar dm³K-1mol-1)
1) 8.33 bar
2) 9.33 bar
3) 7.33 bar
4) 6.33 bar
Answer:
2) 9.33 bar
Solution:
Given data: V = 5dm³ = 5 × 10-3m³
T = 27 + 273K = 300K
R = 8.314Pam³k-1mole-1

Total no.of moles n = 0.125 + 1.5 + 0.25 = 1.875

Question 21.
Pay load is defined as the difference between the mass of displaced air and the mass of the balloon. Calculate the pay load when a balloon of radius 10m, mass 100kg is filled with helium at 1.66 bar at 27°C. (Density of air = 1.2kgm-3 and R = 0.083 bar dm³K-1mol-1).
1) 3607.6 kg
2) 3707.6 kg
3) 3807.6 kg
4) 3507.6 kg
Answer:
3) 3807.6 kg
Solution:
Pay load is the difference between the mass of displaced air and the mass of the ballon
Given that r = 10m
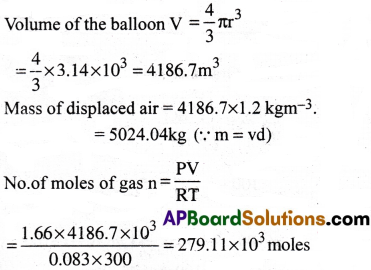
Mass of helium = 279.11 × 10³ × 4 = 1116.4kg
Mass of lull balloon = 100 + 1116.4 = 1216.4kg
∴ Pay load
= Mass of displaced Air- Mass of balloon
= 5024.04 – 1216.44 = 3807.6kg
Question 22.
Calculate the volume occupied by 8.8g of CC>2 at 31.I°C and 1 bar pressure.
R = 0.083 bar dm³K-1mol-1.
1) 5.25 L
2) 5.05 L
3) 5.35 L
4) 5.45 L
Answer:
2) 5.05 L
Solution:
Given data: w = 8.8g
Molecular weight of CO2, M = 44
T = 31.1°C + 273 = 304.1K
Pressure p = 1bar
Gas constant R = 6.083 bar dm³K-1mol-1.
No. of moles of CO2, n = \(\frac{8.8}{44}\) = 0.2
From ideal gas equation,

Question 23.
2.9g of Ag as at 95°C occupied the same volume as 0.184g of dihydrogen at 17°C, at the same pressure. What is the molar mass of the gas?
1) 30 g/mol
2) 40 g/mol
3) 50 g/mol
4) 60 g/mol
Answer:
2) 40 g/mol
Solution:
Gas equation is PV = nRT
When volume and pressure of two gases are same, then
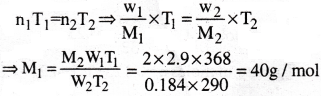
Question 24.
A mixture of dihydrogen and dioxygen at one bar pressure contains 20% by weight of dihydrogen. Calculate the partial pressure of dihydrogen.
1) 0.8 bar
2) 0.7 bar
3) 0.6 bar
4) 0.9 bar
Answer:
1) 0.8 bar
Solution:
Weight of H2 = 20g
Mol. Weight of H2 = 2
Moles of H2 nH2 = \(\frac{20}{2}\) = 10
Weight of O2 = 100 – 20 = 80g
Mol. Weight of O2 = 32
∴ Moles of O2 nO2 = \(\frac{80}{32}\) = 2.5
Total moles, n = n1 + n2 = 10 + 2.5 = 12.5
Total pressure, P = 1 bar
Partial pressure of H2
= \(\frac{\mathrm{n}_{\mathrm{H}_2}}{\mathrm{n}}\) × P = \(\frac{10}{12.5}\) × 1 = 0.8 Bar
![]()
Question 25.
What would be the SI unit for the quantity pV²T²/n?
1) Nm³K²mole-1
2) Nm²K4mole-1
3) Nm²K²mole-1
4) Nm4K²mole-1
Answer:
4) Nm4K²mole-1
Solution:
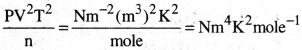
Question 26.
In terms of Charles law the lowest possible temperature is.
1) 0°C
2) 273°C
3) -273°C
4) 786°C
Answer:
3) -273°C
Solution:
At -273°C, Volume of the gas becomes equal to zero i.e., the gas does not exist.
Question 27.
Critical temperature for carbondioxide and methane are 31.1°C and -81.9°C respectively. Which of these has stronger intermolecular forces and why?
1) CH4
2) CO2
3) CO2 & CH4
4) None of these
Answer:
2) CO2
Solution:
Higher the critical temperature, more easily the gas can be liquified i.e. greater are the inter molecular forces of attraction.
Therefore, CO2 has stronger inter molecular forces than CH4.
Question 28.
Air is cooled from 25°C to 0°C. Calculate the decrease in rms speed of the molecules.
1) 4 %
2) 5 %
3) 6 %
4) 7 %
Answer:
1) 4 %
Solution:
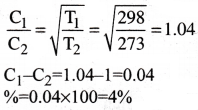
Question 29.
Find the rms, most probable and average speeds of SO2 at 27°C.
1) 3.42 × 104cm/s, 2.79 × 104cm/s, 3.15 × 104cm/s
2) 3.41 × 104cm/’s, 2.78 × 104cm/s, 4.15 × 104cm/s
3) 4.42 × 104cm/s, 2.79 × 104cm/s, 3.15 × 104cm/s
4) 3.42 × 104cm/s, 3.79 × 104cm/s, 3.14 × 104cm/s
Answer:
1) 3.42 × 104cm/s, 2.79 × 104cm/s, 3.15 × 104cm/s
Solution:
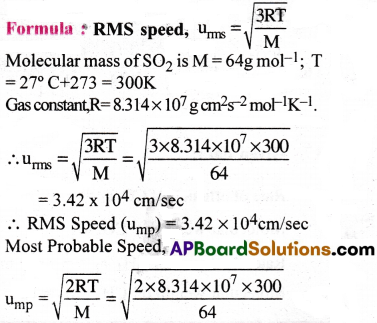
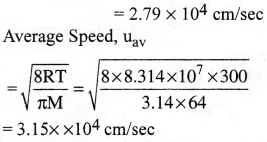
Question 30.
Calculate RMS speed, most probable speed and average speed of Oxygen at 27°C.
1) 5.835 × 104cm/s, 3.848 × 104cm/s, 4.555 × 104cm/s
2) 4.845 × 104cm/s, 4.984 × 104cm/s, 4.455 × 104cm/s
3) 4.835 × 104cm/s, 3.948 × 104cm/s, 4.455 × 104cm/s
4) 4.735 × 104cm/s, 3.948 × 104cm/s, 5.445 × 104cm/s
Answer:
3) 4.835 × 104cm/s, 3.948 × 104cm/s, 4.455 × 104cm/s
Solution:
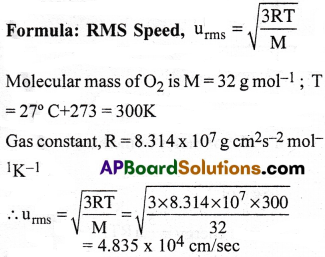
∴ RMS Speed (ump) = 4.835 × 104cm/sec
Most Probable Speed,
ump = 0.8166 × RMS Velocity
= 0.8166 × 4.835 × 104
= 3.948 × 104 cm/sec
Average Speed,
uav = 0.9213 × RMS Velocity
= 0.9213 × 4.835 × 104 = 4.455 × 104 cm/sec
Problems on Boyle’s Law:
Question 31.
A balloon is filled with hydrogen at room temperature. It will burst if pressure ex¬ceeds 0.2 bar. If at 1 bar pressure the gas occupies 2.27 L volume, upto what volume can the balloon be expanded?
1) 11.35 L
2) 12.25 L
3) 13.45 L
4) 14.15 L
Answer:
1) 11.35 L
Solution:
Given data:
Initial Pressure P1 = 1 bar
Initial volume V1 = 2.27 L
Final Pressure P2 = 0.2 bar
Final volume V2 = ?
From Boyle’s law, P1V1 = P2V2
![]()
Problems on charle’s Law:
Question 32.
On a ship sailing in pacific ocean where temperature is 23.4°C, a balloon is filled with 2 L air. What will be the volume of the balloon when the ship reaches Indian ocean, where temperature is 26.1°C? [AP 20]
1) 2.118 L
2) 2.008 L
3) 2.018 L
4) 2.228 L
Answer:
2) 2.008 L
Solution:
Given data:
Initial Temperature T1 = (23.4 + 273)K = 296.4 K
Initial volume V1 = 2 L
Final Temperature T2 = (26.1 + 273)K = 299.1 K
Final volume V2 = ?
From Charles law, \(\frac{V_1}{T_1}=\frac{V_2}{T_2}\)
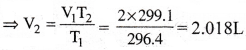
![]()
Question 33.
At 25°C and 760 mm of Hg pressure a gas occupies 600 ml volume. What will be its pres-sure at a height where temperature is 10°C and volume of the gas is 640 ml.
1) 667.6 mm
2) 676.6 mm
3) 666.6 mm
4) 676.9 mm
Answer:
2) 676.6 mm
Solution:
Given data:
P1 = 760 mm, V1 = 600 ml
T1 = (25 + 273)K = 298K
V2 = 640 ml, T2 = (10 + 273)K = 283K
P2 = ?

Problems on Graham’s law:
Question 34.
360 cm³ of CH4 gas diffused through n porous membrane in 15 minutes. Under similar conditions, 120 cm³ of another gas diffused in 10 minutes. Find the molar mass of the gas. [AP 18]
1) 64g.mol-1
2) 62g.mol-1
3) 63g.mol-1
4) 65g.mol-1
Answer:
1) 64g.mol-1
Solution:
Rate of diffusion of CH4,
r1 = \(\frac{v}{t}=\frac{360}{15}\) = 24 cm³/min
Molecular weight of CH4 is M1 = 16
Rate of diffusion of unknown gas,
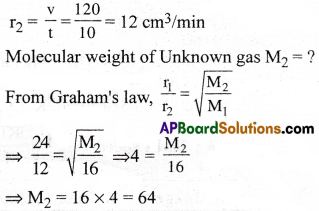
Molar mass of unknown gas = 64.mol-1
Question 35.
Carbon dioxide and another gas ‘X’ have their rates of diffusion as 0.290ccs-1 and 0.271cc.s-1 respectively. Find the vapour density of the gas ‘X’ if the vapour density of carbon dioxide is 22.
1) 25.17
2) 25.18
3) 25.19
4) 25.20
Answer:
3) 25.19
Solution:
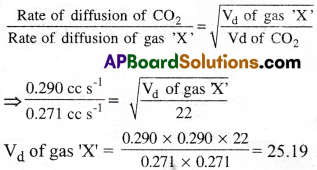
Question 36.
A neon-dioxygcn mixture contains 70.6g dioxygen and 167.5 g of neon. If pressure of the mixture of gases in the cylinder is 25 bar. What is the partial pressure of dioxygen and neon in the mixture?
1) 5.35 bar, 19.55 bar
2) 5.25 bar, 19.75 bar
3) 5.45 bar, 19.45 bar
4) 5.55 bar, 19.55 bar
Answer:
2) 5.25 bar, 19.75 bar
Solution:
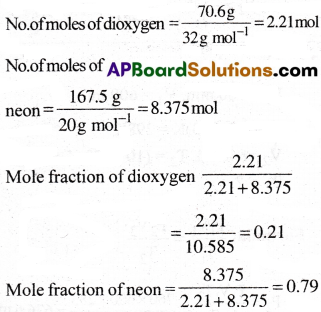
Mole fraction of neon = 1 – 0.21 = 0.79
Partial pressure
= Mole fraction × total pressure
Partial pressure of oxygen
= 0.21 × (25bar) = 5.25 bar
Partial pressure of neon
= 0.79 × (25bar) = 19.75 bar
![]()
Question 37.
Find RMS Speed, average speed and most probable speed of CO2 at 27°C.
1) 4.12 × 10² m/s–, 3.8 × 10² m/s-1, 3.36 × 10² m/s-1
2) 4.11 × 10² m/s–, 3.7 × 10² m/s-1, 3.35 × 10² m/s-1
3) 4. 10 × 10² m/s–, 3.8 × 10² m/s-1, 3.36 × 10² m/s-1
4) 4.12 × 10² m/s–, 3.8 × 10² m/s-1, 3.35 × 10² m/s-1
Answer:
1) 4.12 × 10² m/s–, 3.8 × 10² m/s-1, 3.36 × 10² m/s-1
Solution:
Given data:
Molecular mass of CO2 is M = 44g mol-1;
T = (27 + 273)K = 300K
Gas constant R = 8.314 Jmol-1K-1.
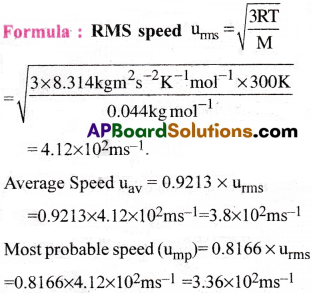
Question 38.
Calculate kinetic energy of 5c moles of Nitrogen at 27°C. |AP’18||TS 15,171
1) 18006.50 J
2) 18506.50 J
3) 18706.50 J
4) 18606.50 J
Answer:
3) 18706.50 J
Solution:
Given data:
No.of moles of n = 5 moles
T = (27 + 273)K = 300 K.
R = 8.314 Jmol-1k-1
Kinetic Energy = \(\frac{3}{2}\) n RT
= \(\frac{3}{2}\) × 5 × 8.314 × 300 = 18706.50J
Question 39.
Calculate Kinetic energy (in SI units ) of 4 g. of methane at -73°C. [AP, TS 19]
1) 633.6 J
2) 613.6 J
3) 643.6 J
4) 623.6 J
Answer:
4) 623.6 J
Solution:
Given data:
T = (-73 + 273 )K = 200 K
R = 8.314 J mol-1 K-1
n = No. of moles of methane
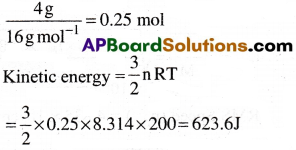
Question 40.
Calculate the ratio of kinetic energies of 3g of hydrogen and 4g of oxygen at given temperature. [TS 16, 18, 20][AP 19]
1) 10 : 1
2) 11 : 1
3) 12 : 1
4) 14 : 1
Answer:
3) 12 : 1
Solution:
Since the temperature is constant;

Question 41.
Critical temperatures of ammonia and carbon dioxide are 405.5K and 304.10 K respectively. Which of these gases will liquify first when you start cooling from 500K to their critical temperature?
1) Ammonia
2) Carbon dioxide
3) Both 1 & 2
4) None of these
Answer:
1) Ammonia
Solution:
Ammonia will liquify first because its critical temperature will be reached first. Liquefaction of CO2 will require more cooling. Gases with higher critical temperature Liquify easily.
![]()
Question 42.
Choose the correct option for graphical representation of Boyle’s law, which shows a graph of pressures vs volume of a gas at different temperatures.
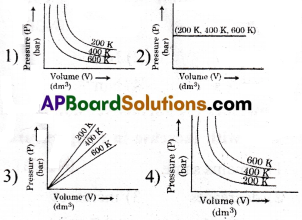
Answer:
4
Question 43.
A person living in Shimla observed that cooking food without using pressure cooker takes more time. The reason for this observation is that at high altitude
1) pressure increases
2) temperature decreases
3) pressure decreases
4) temperature increases
Answer:
3) polarisability of interacting particles
Question 44.
The volume occupied by 1.8g of water vapour at 374°C and 1 bar pressure will be [Use R = 0.083bar L.K-1mol-1)
1) 96.66 L
2) 55.87 L
3) 3.10 L
4) 5.37 L
Answer:
4) 5.37 L
Question 45.
What is the density of N2 gas at 227°C and 5.00 atm pressure?
(R = 0.082L atm K-1 mol-1!
1) 1.40 g/ mL
2) 2.81 g/mL
3) 3.41 g/mL
4) 0.29 g/mL
Answer:
3) 3.41 g/mL
Question 46.
As the temperature increases, average kinetic energy of molecules increases. What would be the effect of increase of temperature on pressure provided the volume is constant?
1) increases
2) decreases
3) remains same
4) becomes half
Answer:
1) increases
![]()
Question 47.
Equal moles of hydrogen and oxygen gases are placed in a container with a pin hole through which both can escape, What fraction of the oxygen escapes in the time required for one-half of the hydrogen to escape?
1) 3/8
2) 1/2
3) 1/8
4) 1/4
Answer:
3) 1/8
Question 48.
Choose the correct option for the total pressure (in atm) in a mixture of 4g O2 and 2g H2 confined in a total volume of one litre at 0°C is [Given R = 0.0822L atom mol-1K-1, T = 273K]
1) 26.032
2) 2.518
3) 2.602
4) 25.18
Answer:
4) 25.18
Question 49.
The pressure of a 1:4 mixture of dihydrogen and dioxygen enclosed in a vessel is one atmosphere. What would be the partial pressure of dioxygen?
1) 0.8 × 105 atm
2) 0.008Nm-2
3) 8 × 104 Nm-2
4) 0.25 atm
Answer:
3) 8 × 104 Nm-2
Question 50.
A mixture of N2 and Ar gases in a cylinder contains 7g of N2 and 8 g of Ar. If the total pressure of the mixture of the gases in the cylinder is 27 bar, the partial pressure of N2 is :
[Use atomic masses (in g mol-1) : N = 14, Ar = 40]
1) 9 bar
2) 12 bar
3) 15 bar
4) 18 bar
Answer:
3) 15 bar
Question 51.
By what factor does the average velocity of a gaseous molecule increase when the temperature (in Kelvin) is doubled?
1) 2.0
2) 2.8
3) 4.0
4) 1.4
Answer:
4) 1.4
![]()
Question 52.
A gas at 350K and 15 bar has molar volume 20 percent smaller than that for an ideal gas under the same conditions. The correct option about the gas and its compressibility factor(Z) is
1) Z<1 and repulsive forces are dominant 2) Z>1 and attractive forces are dominant
3) Z>1 and repulsive forces are dominant
4) Z<1 and attractive forces are dominant
Answer:
4) Z<1 and attractive forces are dominant
Question 53.
Given vanderWaal’s constant for NH3, H2, O2 and CO2 are respectively 4.17, 0.244, 1.36 and 3.59. Which one of the following gases is most easily liquefied?
1) NH3
2) H2
3) O2
4) CO2
Answer:
1) NH3
Question 54.
Which curve in adjacent figure represents the curve of ideal gas?
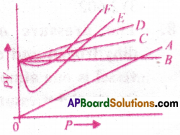
1) B only
2) C and D only
3) E and F only
4) A and B only
Answer:
1) B only
Question 55.
Which of the following property of water can be used to explain the spherical shape of rain droplets?
1) viscosity
2) surface tension
3) critical phenomena
4) pressure
Answer:
2) surface tension
Question 56.
Increase In kinetic energy can overcome intermodular forces of attraction. How will the viscosity of liquid be affected by the increase in temperature?
1) Increase
2) No effect
3) Decrease
4) No regular pattern will be followed
Answer:
3) Decrease
Question 57.
What is SI unit of viscosity coefficient (η)?
1) Pascal
2) Nsm-2
3) km-2s
4) Nm-2
Answer:
2) Nsm-2
Question 58.
How does the surface tension of a liquid vary with increase in temperature?
1) remains same
2) decreases
3) increases
4) no regular pattern is followed
Answer:
2) decreases
Question 59.
Dipole-dipole forces act between the molecules possessing permanent dipole. Ends of dipoles possess ‘partial charges’. The partial charge is
1) more than unit electronic charge
2) equal to unit electronic charge
3) less than unit electronic charge
4) double the unit electronic charge
Answer:
3) less than unit electronic charge
![]()
Question 60.
The interaction energy of London force is inversely proportional to sixth power of the distance between two interacting particles but their magnitude depends upon
1) charge of interacting particles
2) mass of interacting particles
3) polarisability of interacting particles
4) strength of permanent dipoles in the particles.
Answer:
3) polarisability of interacting particles