Solving AP 10th Class Physical Science Model Papers and AP 10th Class Physical Science Question Paper April 2023 regularly is an effective strategy for time management during exams.
AP SSC Physical Science Question Paper April 2023 with Solutions
Time: 2 Hours
Maximum Marks: 50
Instructions:
- The question paper consists of 4 sections and 17 questions.
- Internal choice is available only for Q.No.12 in section III and for all the questions in section IV.
- In 2 hours, 15 minutes are allotted to read the question paper.
- All answers shall be written in the answer booklet only.
- Answers shall be written neatly and legibly.
Section-I
(8 × 1 = 8 Marks)
Note:
- Answer ALL the questions.
- Each question carries 1 mark.
Question 1.
Ask a question about temperature.
Answer:
What is temperature?
Question 2.
A light ray enters from rarer medium A to denser medium B as shown in the figure.
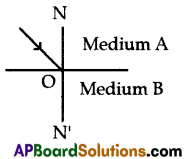
Then complete the path of light in medium B and draw the whole diagram in your answer sheet.
Answer:
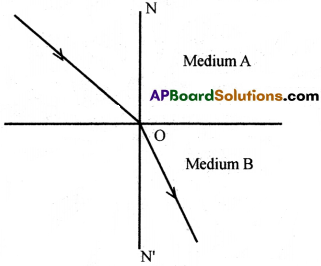
Question 3.
The electronic configuration of an element is 1s22s22p4, then to which period does it belong?
Answer:
Second
![]()
Question 4.
The electronic configuration of an element ‘X’ is 2, 8. Then write several valency electrons.
Answer:
Eight
Question 5.
Name the device that converts electrical energy into mechanical energy.
Answer:
Electric Motor
Question 6.
Write any one daily life application of the thermite process.
Answer:
To join the railway tracks.
(OR)
To join the cracked machine parts.
Question 7.
What gas is produced when magnesium reacts with hydrochloric acid?
Answer:
Hydrogen
Question 8.
Draw the diagram of a lens that will be recommended by an eye doctor to a long-sighted patient.
Answer:
The doctor recommends a convex lens to a long-sighted patient.
Section-II
(3 × 2 = 6 Marks)
Note:
- Answer all the questions.
- Each question carries 2 marks.
Question 9.
What is the lens formula? Explain the terms in it.
Answer:
\(\frac{1}{f}=\frac{1}{v}-\frac{1}{u}\)
Here, f = Focal length;
u = Distance of object;
v = Distance of image
Question 10.
Predict which element has a larger atomic size between Na and Na+. Why?
Answer:
- Na has a larger atomic size than Na+.
- The nucleus of the Na+ ion attracts outer shell electrons with stronger nuclear force than that of the Na atom. So, the Na+ ion shrinks in size to Na atom.
![]()
Question 11.
Ask any two questions to understand “magnetic field lines”.
Answer:
- Are magnetic lines closed?
- Will they intersect each other?
- What is the direction of magnetic field lines?
Section-III
(3 × 4 = 12 Marks)
Note:
- Answer all the questions.
- Each question carries 4 marks.
Question 12.
Draw any one of the following diagrams.
(A) Draw the shapes of s and p orbitals.
(B) Draw the circuit arrangement indicating the comparison of resistances of different materials having the same length and area of cross-section. Different metal rods are connected between P and Q.
Answer:
(A)
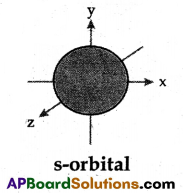
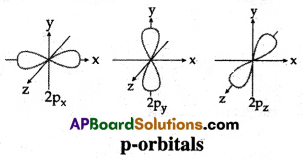
(B)
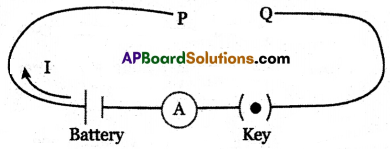
P, Q: Gap for placing different materials.
I: Electric Current
A: To measure current in an electric circuit (Ammeter)
K: On and off switch.
Question 13.
Some ores and their formulae are given in the following table.
| Ore | Formula |
| Bauxite | Al2O3.2H2O |
| Magnesite | MgCO3 |
| Epsom Salt | MgSO4.7H2O |
| Haematite | Fe2O3 |
| Galena | PbS |
| Gypsum | CaSO4.2H2O |
Answer the following questions using the above table.
(a) Galena is an ore of _______ metal.
(b) Write an ore of iron.
(c) Which is carbonate ore from the above table?
(d) How many water molecules are present in Epsom salt?
Answer:
(a) Lead (or) Pb
(b) Haematite (or) Fe2O3
(c) Magnesite (or) MgCO3
(d) Seven
![]()
Question 14.
What is the reason behind the shining of diamonds and how do you appreciate it?
Answer:
- Total internal reflection.
- Diamonds have a high refractive index value, (n = 2.42)
- The critical angle of. diamond is very less due to high refractive index. (C = 24.4°)
- For the above reasons, diamonds are famous as precious stones.
- So, I appreciate the role of total internal reflection that makes diamonds precious stones.
Section-IV
(3 × 8 = 24 Marks)
Notes:
- Answer all the questions.
- Each question carries 8 marks.
- Each question has an internal choice.
Question 15.
(A) Write any four differences between evaporation and boiling.
(OR)
(B) Define the eye defect ‘Myopia’ and explain the process of correction.
Answer:
(A) Evaporation:
- The process of escaping molecules from the surface of a liquid at any temperature is called evaporation.
- Evaporation takes place at any temperature.
- It is a cooling process.
- It is a surface phenomenon.
Boiling:
- The process in which the liquid phase changes to a gaseous phase at a constant temperature is called boiling.
- Boiling takes place at a constant temperature.
- It is a heating process.
- It is a bulk phenomenon.
(OR)
(B) (i) Some people cannot see objects at long distances but can see near objects. This type of eye defect in vision is called myopia.
(ii) In people suffering from myopia the rays coming from distant objects after refraction through the eye lens form an image before the retina.
(iii) By using a biconcave lens we can rectify myopia.
(iv)

Question 16.
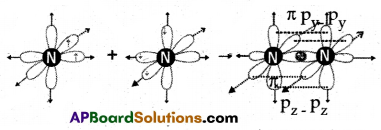
(A) Explain the formation of the ‘N2‘ molecule.
(OR)
(B) Explain the cleansing action of soap.
Answer:
(A) 1. The atomic number of Nitrogen is 7.
2. The electronic configuration is 1s22s22p3.
3. Nitrogen has three unpaired electrons in the ‘p’ orbital.
4. When two nitrogen atoms approach each other, the px orbital of one nitrogen atom overlaps the px orbital of another nitrogen atom giving σpx – px bond along the internuclear axis.
5. The py and pz orbitals of one ‘N’ atom overlap the py and pz orbital of the other ‘N’ atom laterally, respectively perpendicular to the inter-nuclear axis giving πpy – py, and πpz – pz bonds. Therefore, the N2 molecule has a triple bond between two nitrogen atoms.
(OR)
(B) 1. Suppose that we put the dirty cloth in the soap solution. Dirt is mainly greasy matter.
2. The soap molecules are arranged radially with hydrocarbon ends directed inwards into the greasy matter and ionic part directed outwards into water.

3. When a dirty cloth is inserted in the solution then the hydrocarbon part sticks to the dirt or oil. With a little agitation, the dirt particles get entrapped by the soap micelles and get dispersed in water.
4. Due to which the soap water gets dirty and the cloth gets cleaned.
5. We know that soaps and detergents make oil and dirt present on the cloth come out into the water, thereby making the cloth clean.
Another Explanation:
1. When soap is dissolved in water, its hydrophobic ends attach themselves to dirt and remove it from the cloth.
2. The hydrophobic end of the soap molecules moves towards the dirt or grease particle.
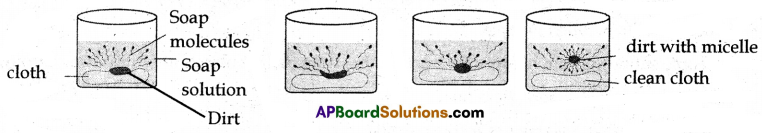
3. The hydrophobic ends attached to the dirt particle and try to pull out. The molecules of soap surround the dirt particles at the center of the cluster and form a spherical structure called micelle.
4. These micelles remain suspended in water-like particles in a colloidal solution.
5. The various micelles present in water do not come together to form a precipitate as each micelle repels the other because of the ion-ion repulsion.
6. Thus, the dust particles remain trapped in micelles (which remain suspended) and are easily rinsed away with water. Hence, soap micelles remove dirt by dissolving it in water.
![]()
Question 17.
(A) State Ohm’s law. Suggest an experiment to verify it and explain the procedure.
(OR)
(B) What is the water for the crystallization of salts? Suggest an activity to explain it.
Answer:
(A) Ohm’s Law: The current through the conductor element is proportional to the potential difference applied between its ends provided the temperature remains constant.
V ∝ I ⇒ V = IR
Aim: To verify Ohm’s law
Materials Required: 6V Battery eliminator, 0 to 1A Ammeter, 0-6V voltmeter, copper wires, 50 cm manganin coil, Rheostat, switch and 3V LED, etc.
Procedure:
1. Connections are made as shown in the figure.
2. By changing the position of the rheostat, change the flow of current in the circuit.
3. Note the reading in the voltmeter and ammeter and tabulate the values below.
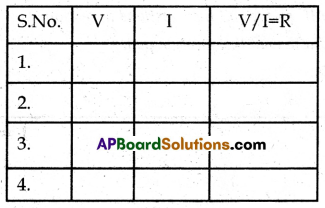
4. From the above table we observe that V/I = Constant.
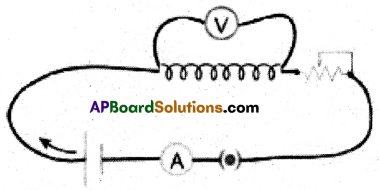
5. Hence, Ohm’s law is verified.
(OR)
(B) Water of Crystallization:
(i) The water molecules present in one formula unit of salt crystals are called water of crystallization.
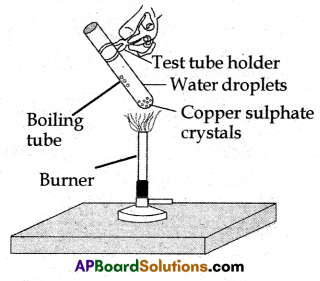
Activity:
- Take a few crystals of copper sulfate in a dry test tube and heat them.
- We observe the water droplets appear on the inner walls of the test tube.
- This is due to the evaporation of water of crystallization present in crystals.
- Blue colour copper sulphate turns into white. When water is added to crystals, copper sulphate regains its blue colour.