Solving AP 10th Class Physical Science Model Papers Set 6 regularly is an effective strategy for time management during exams.
AP SSC Physical Science Model Paper Set 6 with Solutions
Time: 2 Hours
Maximum Marks: 50
Instructions:
- The question paper consists of 4 sections and 17 questions.
- Internal choice is available only for Q.No.12 in section III and for all the questions in section IV.
- In 2 hours, 15 minutes is allotted to read the question paper.
- All answers shall be written in the answer booklet only.
- Answers shall be written neatly and legibly.
Section-I
(8 × 1 = 8 Marks)
Note:
- Answer all the questions.
- Each question carries 1 mark.
Question 1.
In which process of metallurgy, the molten metal is stirred with logs of green wood?
Answer:
Poling Process
Question 2.
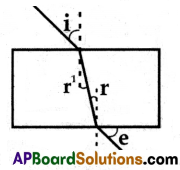
Which is wrongly marked in the given diagram?
Answer:
e
Question 3.
What is humidity?
Answer:
The amount of water vapour present in air is called humidity.
![]()
Question 4.
What is the effective resistance of an appliance marked “240V, 4A”?
Answer:
60 Ω
Question 5.
Cl, X, I an example of triad. What is ‘X’?
Answer:
Br
Question 6.

In the diagram ‘X’ is?
Answer:
Hydrophobic End
Question 7.
Draw a diagram showing the refraction of a ray of light through a glass prism and mark the angle of deviation.
Answer:

Question 8.
Fill in the following boxes.
| No. of Protons | No. of Neutrons | Atomic Number | Symbol | Valency |
| 8 | 9 | ? | ? | ? |
Answer:
Atomic Number: 8
Symbol: 8O17
Valency: 2
Section-II
(3 × 2 = 6 Marks)
Note:
- Answer ALL the questions.
- Each question carries 2 marks.
Question 9.
Suppose you are inside the water in a swimming pool. Your friend is standing on the edge. Do you find your friend taller or shorter than his actual height? Why?
Answer:
My friend appears to be taller than his actual height for me. When I saw him from water, the light travels from a denser medium (water) to a rarer medium (air). So it bends away from the normal (refraction of light). So he appears to be taller.
![]()
Question 10.
Which atom is bigger in size, Ne or Ar? Why?
| “0 Group” |
|
He |
Answer:
Ar, In groups, as we go down number of shells increases.
Question 11.
Why do sometimes cooking vessels get blackened on a gas or kerosene stove?
Answer:
Sometimes cooking vessels get blackened on a gas or kerosene stove because the air’s inlets get blocked, so the fuel gases do not completely undergo combustion.
Section-III
(3 × 4 = 12 Marks)
Note:
- Answer ALL the questions.
- Each question carries 4 marks.
Question 12.
Draw any one of the following diagrams:
(A) Write the characteristics of the images formed by a convex lens having a focal length of 25 cm, when an object is kept on the principal axis at a distance of 50 cm and 75 cm.
(B) Draw a universal pH value indicator and identify different substances.
Answer:
(A) Focal length (f) = 25 cm
(i) Object distance (u) = 50 cm = 2f cm
Here the object is placed at ‘2f’ (2F2) in front of a convex lens.
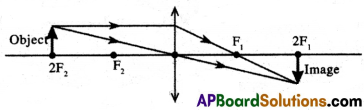
The characteristics of the image are Real, Inverted, the Same size as the object, and Formed at a ‘2f’ (2F1) distance on the other side of the lens.
(ii) Object distance u = 75 cm > 2f
Here object is placed beyond ‘2f’ in front of a convex lens.
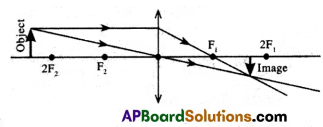
The characteristics of the image are Real and inverted, the size of the image is less than that of the object, and the Image is formed between f and 2f (F1 and 2F1) on another side of the lens.
(B)
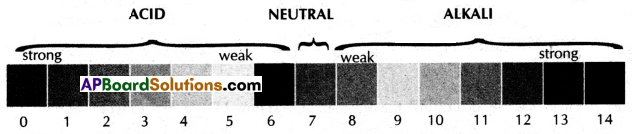
![]()
Question 13.
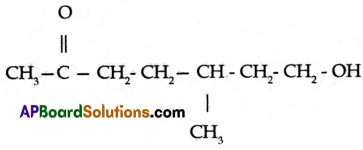
Observe the structure and answer the following.
(a) Write the name of the principal functional group present in the compound.
(b) Identify the parental chain in the compound.
(c) What are the substituents in the above compound?
(d) Name the above compound as per IUPAC nomenclature.
Answer:
(a) Ketone
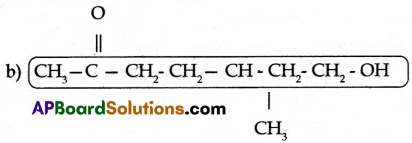
(c) Methyl group, Hydroxy group
(d) 7- hydroxy – 5 – methyl heptan – 2 – one
Question 14.
An element has the atomic number 17. Where would you expect this element in the Periodic Table? Why?
Answer:
Electronic configuration of the given element is 1s2 2s2 2p6 3s2 3p5. So, it is in the 3rd period and 17th group of the periodic table. Due to the valency electronic configuration of 3s2 3p5, it belongs to the 3rd period and 17th group.
Section-IV
(3 × 8 = 24 Marks)
Note:
- Answer ALL the questions.
- Each question carries 8 marks.
- Each question has an internal choice.
Question 15.
(A) Explain the formation of the rainbow.
(OR)
(B) Collect information on the working of optical fibers. Prepare a report about various uses of optical fibers in our daily lives.
Answer:
(A) Observe the figure.
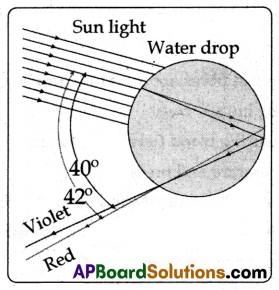
(i) The rays of sunlight enter the drop near its top surface.
(ii) At the first refraction, the white light is dispersed into its spectrum of colours, with violet being deviated the most and red the least.
(iii) Reaching the opposite side of the drop, each colour is reflected into the drop because of total internal reflection.
(iv) Arriving at the surface of the drop, each colour is again refracted into the air.
(v) At the second refraction the angle between red and violet rays further increases when compared to the angle between those at first refraction.
(vi) The angle between the incoming and outgoing rays can be anything between 0° and about 42°.
(vii) We observe a bright rainbow when the angle between incoming and outgoing rays is near the maximum angle of 42°. Diagrammatically it is shown in the figure.
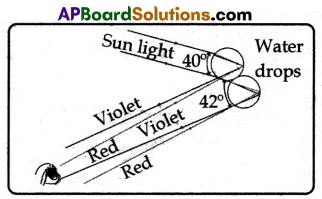
(viii) Although each drop disperses a full spectrum of colours, an observer is in a position to see only a single colour from any one drop depending upon its position.
(ix) If violet light from a single drop reaches the eye of an observer, red light from the same drop can’t reach his eye. It goes elsewhere possibly downwards of the eye of the observer. See the figure.
(x) To see red light, one must look at the drop higher in the sky.
(xi) The colour red will be seen when the angle between a beam of sunlight and light sent back by a drop is 42°.
(xii) The colour violet is seen when the angle between a sunbeam and light sent back by a drop is 40°.
(xiii) If you look at an angle between 40° and 42°, you will observe the remaining colours of VIBGYOR.
(OR)
(B) Principle: The basic principle for working of optical fiber is T.I.R (Total Internal Reflection)
Description: It is a very thin fiber made of glass quartz (or) plastic having a radius of about a micrometer (10-6 m). Each fibre consists of a core (n1) and cladding (n2). A bunch of thin fibers forms a light pipe.

Working: The light going into the pipe makes a nearly glancing incident on the wall. The angle of incidence is greater than the critical angle and hence T.I.R takes place. In this way, light is transmitted.
Uses:
- Medical investigation: Optical fibers are used in laparoscopes, and endoscopes for visual examination of inaccessible regions in the human body.
- Photometric sensors: Measuring blood flow in the heart.
- Sensors: To measure temperature and pressure.
- Refractometers: To determine the refractive indices of liquids.
- Communication: Different telephone signals by superposing on the optical beam can be transmitted through fibers without any interference.
Question 16.
(A) What is the refining of metals? Explain some methods of refining.
(OR)
(B) Explain (i) the Aufbau principle and (ii) Hund’s rule with an example each.
Answer:
(A) The process of obtaining pure metal from the impure is called refining of the metal.
1. Distillation:
- This method is very useful for the purification of low-boiling metals like zinc and mercury.
- The extracted metal in the molten state is distilled and the pure metal as distillate is obtained.
2. Poling:
- The molten metal is stirred with logs (poles) of greenwood.
- The impurities are removed either as gases or they get oxidized and form scum (slag) over the surface of the molten metal.
- Blister copper is purified by this method.
- The reducing gases, evolved from the wood, prevent the oxidation of copper.
3. Liquation:
In this method, a low melting metal like Tin can be made to flow on a sloppy surface to separate it from high-melting impurities.
(OR)
(B) In the ground state the electronic configuration can be built up by placing electrons in the lowest available orbitals until the total number of electrons added is equal to the atomic number. This is called the Aufbau principal.
Two general rules help us to predict electronic configurations.
(i) Electrons are assigned to orbitals in order of increasing value of (n + l)
Eg: for 2s orbital (n + l) = 2 + 0 = 2
for 3s orbital (n + l) = 3 + 0 = 3
Hence, 2s has lower energy than 3s orbital.
(ii) For subshells with the same value of (n + l), electrons are assigned first to the subshell with a lower ‘n’ value.
Eg: (n + l) value of 2p = 2 + 1 = 3
(n + l) value of 3s = 3 + 0 = 3
Here electrons are first assigned to the 2s orbital.
Hund’s rule helps in writing the electronic configuration of an atom.
According to this rule, the orbitals of equal energy are occupied with one electron each before the pairing of electrons starts.
Ex: Oxygen – O
atomic number – 8
Electronic configuration – 1s2 2s2 2p4
According to this rule first, four electrons occupy 1s and 2s orbitals.
Then the remaining four electrons occupy one each in 2px, 2py, and 2pz then pairing takes place in 2px.
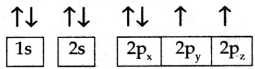
In this way, Hund’s rule helps write the electronic configuration of an atom.
![]()
Question 17.
(A) Write an activity to show that the solutions of compounds like alcohol and glucose do not show acidic character even though they are having Hydrogen.
(OR)
(B) Which quantity determines the direction of heat flow? Experiment to support this.
Answer:
(A) (i) Prepare solutions of glucose and alcohol.
(ii) Fix two iron nails on a rubber cork and place the cork in a beaker as shown in the figure.
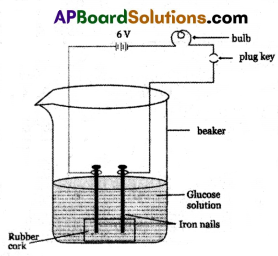
(iii) Connect the nails to the two terminals of a 6-volt DC battery through a switch and a bulb.
(iv) Now pour glucose solution (C6H12O6) and switch on the current.
(v) The bulb does not glow. This shows that glucose solution does not conduct electricity.
(vi) Repeat this experiment with alcohol solution in the beaker. The bulb does not glow again. That means the alcohol solution does not conduct electricity.
(vii) Due to the absence of ions in glucose and alcohol solutions they do not conduct electricity.
(viii) Glucose and alcohol do not dissociate in water to produce H+ ions even though they contain hydrogen.
(ix) Glucose and alcohols are not categorized as acids because they do not produce H+ ions in aqueous solution.
(OR)
Temperature is the quantity that determines the direction of heat flow.
Experiment Procedure:
(i) Take water in a container and heat it to 60°C.
(ii) Take a cylindrical transparent glass jar and fill half of it with this hot water.
(iii) Very gently pour coconut oil over the surface of the water.
(iv) Put a lid with two holes on the top of the glass jar.
(v) Take two thermometers and insert them through the holes of the lid in such a way that the bulb of one thermometer lies only inside the water and the other lies only inside the coconut oil as shown in the figure.
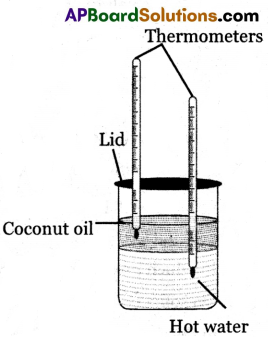
(vi) Observe the readings of the two thermometers. The reading on the thermometer which is kept in water decreases and the reading on the thermometer which is kept in oil increases.
(vii) Here water loses heat energy and oil gains heat energy. Thus heat energy flows from water to oil.
(viii) From this we conclude that heat energy flows from a hotter body to a colder body and also temperature determines the direction of heat flow.