Solving AP 10th Class Physical Science Model Papers and AP 10th Class Physical Science Board Model Paper 2024 regularly is an effective strategy for time management during exams.
AP SSC Physical Science Model Paper 2024 with Solutions
Time: 2 Hours
Maximum Marks: 50
Instructions:
- The question paper consists of 4 sections and 17 questions.
- Internal choice is available only for Q.No.12 in section III and for all the questions in section IV.
- In 2 hours, 15 minutes is allotted to read the question paper.
- All answers shall be written in the answer booklet only.
- Answers shall be written neatly and legibly.
Section-I
(8 × 1 = 8 Marks)
Note:
- Answer ALL the questions.
- Each question carries 1 mark.
Question 1.
Convert 25° C into Kelvin scale.
Answer:
Kelvin scale = Celsius Scale + 273
= 25°C + 273
= 298 K
Question 2.
Give an example of the olfactory indicator.
Answer:
Vanilla Essence, Onion, Clove Oil
Question 3.
From the data given in the table:
| Material Medium | Air | Ice | Water | Diamond |
| Refractive Index | 1.003 | 1.31 | 1.33 | 2.43 |
In which material medium light travels faster?
Answer:
Light travels faster in the air.
![]()
Question 4.
Which material medium is denser in the given figure?

Answer:
Medium B
Question 5.
Draw a neat diagram of the shape of the biconvex lens.
Answer:
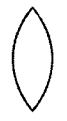
Question 6.
Assertion 1: The Sky appears in blue due to light scattering.
Assertion 2: Blue color has the longest wavelength among all colors of white light.
(A) Both the Assertions are true
(B) Both the Assertions are false
(C) Only Assertion 1 is true
(D) Only Assertion 2 is true
Answer:
(C) Only Assertion 1 is true
Question 7.
Imagine and write the element that exists in the I group and I period.
Answer:
Hydrogen
Question 8.
How do you appreciate the role of soap in daily life?
Answer:
- Soap cleans dirt, germs, and bacteria from the skin.
- Maintains personal hygiene and prevents diseases.
- Removes oil and grease from surfaces.
- Essential for cleaning tasks.
Section-II
(3 × 2 = 6 Marks)
Note:
- Answer ALL the questions.
- Each question carries 2 marks.
Question 9.
A prism with an angle of prism A = 60° produces an angle of minimum deviation of 30°. Find the refractive index of the material of the prism.
Answer:
Given A = 60° and D = 30°
we know,
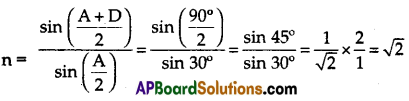
∴ The refractive index of the given prism = \(\sqrt{2}\)
![]()
Question 10.
Pose any two questions to understand the Bohr-Sommerfeld model of an atom.
Answer:
- What are the main postulates of the Bohr-Sommerfeld model?
- How do the allowed orbits in the Bohr-Sommerfeld model depend on the principal quantum number?
- What is the meaning of the quantum numbers in the Bohr-Sommerfeld model?
- How does the Bohr-Sommerfeld model explain the emission and absorption of light by atoms?
Question 11.
Imagine the reason and write why the value of ionization potential II is more than the value of ionization potential I.
Answer:
- The energy required to remove the first electron from the outermost orbit of a neutral gaseous atom of the element is called the first ionization energy.
- The energy required to remove an electron from an unipositive ion is called the second ionization energy.
- The nuclear attraction force on the outermost electron of the unipositive ion is more than the nuclear attraction force on. the outermost electron of a neutral atom.
- Hence, more energy is required to remove an electron from the outermost orbit of an unipositive ion than from a neutral atom. So second ionization energy is higher than the first ionization energy.
Section-III
(3 × 4 = 12 Marks)
Note:
- Answer ALL the questions.
- Each question carries 4 marks.
Question 12.
Draw any one of the following diagrams:
(A) Draw a neat ray diagram of the formation of the image when the object is kept before a convex lens in the following positions:
(i) at F
(ii) between F and P
(B) Draw a neat diagram of the formation of oxygen (O2) molecules.
Answer:
(A) (i) Object is placed at F
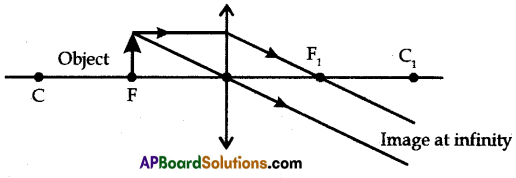
(ii) Object placed between Focal point and P
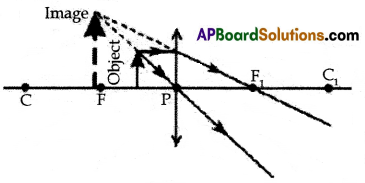
(B)
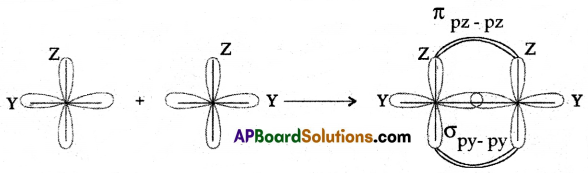
Question 13.
Fill in the given table:
| Sub Shell | Orbital | Number of Orbitals | Maximum Number of Electrons |
| l = 0 | 1 | ||
| l = 1 | P | 6 | |
| l = 3 | 5 | 10 | |
| l = 4 | 7 |
Answer:
| Sub Shell | Orbital | Number of Orbitals | Maximum Number of Electrons |
| l = 0 | s | 1 | 2 |
| l = 1 | p | 6 | 6 |
| l = 3 | d | 5 | 10 |
| l = 4 | f | 7 | 14 |
Question 14.
Write the advantages of parallel and series connections of electric circuits in our daily lives.
Answer:
Advantages of Parallel Connections:
- Maintains constant voltage across devices.
- Devices can operate independently.
- One device’s failure doesn’t affect others.
Advantages of Series Connections:
- Equal flow of current through components.
- Suitable for devices needing the same current.
- Simplicity in design for certain applications.
Section-IV
(3 × 8 = 24 Marks)
Note:
- Answer ALL the questions.
- Each question carries 8 marks.
- Each question has an internal choice.
Question 15.
(A) Define the following:
(1) Dew
(2) Fog
(3) Latent Heat of Vaporization
(4) Latent Heat of Fusion
(OR)
(B) Write about the working nature of the motor.
Answer:
(A) 1. Dew: The water droplets condensed on the surfaces of window panes, flowers, etc., are known as dew.
2. Fog: The thick mist formed by droplets of water keeps on floating in the air and restricts visibility is called fog.
3. Latent Heat of Vapourization: The heat energy required to change 1 gram of liquid to gas at a constant temperature is called the latent heat of vaporization.
4. Latent Heat of Fusion: The heat energy required to convert 1 gm of solid completely into liquid at a constant temperature is called latent heat of fusion.
(OR)
(B) Electric Motor: An electric motor is a device that converts electrical energy into mechanical energy.
Principle: It works on the principle that a current-carrying conductor placed in a magnetic field experiences a force.
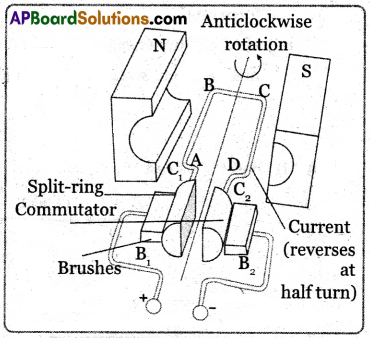
Working Procedure:
- It consists of the armature, strong horseshoe-type magnet, split rings, and carbon brushes.
- Initially let the plane of the coil be in a horizontal position. The split ring C touches the brush B1 and split ring C2 touches the brush B2 when the current flows in the direction ABCD as shown in figure.
- According to the right-hand rule, no force acts on arm CB and DA because as they are parallel to the magnetic field.
- The force acting on the arm AB pushes it downwards while the force acting on the arm CD pushes it upwards. So the armature rotates in the anti-clockwise direction.
- After half a rotation the split ring C comes in contact with brush B2, and C2 in contact with brush B1. So the current in the coil is reversed and flows in the direction of DCBA.
- If the direction of the current in the coil is unchanged the coil gets to and fro motion.
- In an electric motor, the split rings act as a commutator which reverses the direction of the flow of current through a circuit.
- Now the coil rotates continuously in the anti-clockwise direction.
![]()
Question 16.
(A) Write briefly about the following:
(1) Plaster of Paris
(2) Bleaching Powder
(3) Sodium Bicarbonate
(4) Sodium Carbonate
(OR)
(B) Explain the chain reaction when methane is reacted with chlorine to get carbon tetrachloride.
Answer:
(A) 1. Plaster of Paris: Calcium sulphate hemihydrate (CaSO4.\(\frac{1}{2}\)H2O) is called Plaster of Paris. On careful heating of Gypsum (CaSO4.2H2O) at 373K it loses water molecules partially and becomes Plaster of Paris.
CaSO4 \(\frac{1}{2}\)H2O + 1\(\frac{1}{2}\) H2O → CaSO4.2H2O
2. Bleaching Powder: Bleaching powder is produced by the action of chlorine on dry slaked lime (Ca(OH)2). Its formula is CaOCl2.
Ca(OH)2 + Cl2 → CaOCl2 + H2O
- Chlorine is produced during the electrolysis of aqueous sodium chloride (brine).
- This chlorine gas is used for the manufacture of bleaching powder.
- Bleaching power is produced by the action of chlorine on dry-slaked lime [Ca(OH)2].
- Bleaching powder is represented by formulaCaOCl2, though the actual composition is quite complex.
3. Sodium hydrogen carbonate (or) Sodium bicarbonate:
- Sodium hydrogen carbonate is also an ingredient in antacids. Being alkaline, it neutralizes excess acid in the stomach and provides relief.
- It is also used in soda-acid fire extinguishers.
- It acts as a mild antiseptic.
4. Sodium Carbonate:
- Recrystallization of sodium carbonate gives washing soda. It is also a basic salt.
Na2CO3 + 10H2O → Na2CO3.10H2O - Sodium carbonate, (washing soda) is used in glass, soap, and paper industries.
- It is used in the manufacture of sodium compounds such as borax.
- Sodium carbonate can be used as a cleaning agent for domestic purposes.
- It is used for removing the permanent hardness of water.
(OR)
(B) When direct halogenation takes place in alkanes, in the presence of sunlight (hυ) all the hydrogens of that compound will be replaced by halogens.
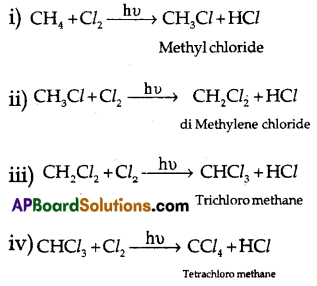
Question 17.
(A) Write the lab activity to understand lateral shift using glass lab.
(OR)
(B) Write an activity to understand the corrosion of iron(rusting) in the presence of water and air.
Answer:
(A) Aim: To find the lateral shift.
Material Required: Drawing board, chart paper, clamps, scale, pencil, thin glass slab, and pins.
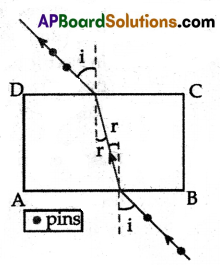
Procedure:
- Clamp a chart paper on a drawing board.
- Place a glass slab in the middle of the paper and draw a borderline along the edges of the slab by using a pencil.
- Remove the slab and name the vertices of the rectangle formed as A, B, C, and D.
- Draw a perpendicular at a point on the longer side (AB) of the rectangle.
- Again keep the slab on paper such that it coincides with the sides of the rectangle ABCD.
- Take two pins and stick them on the perpendicular line to AB.
- Take two more pins and stick them on the other side of the slab in such a way that all pins come to view along a straight line.
- Remove the slab from its place, take out the pins, and draw a straight line by using the dots formed by the pins such that it reaches the first edge (AB) of the rectangle.
- It forms a straight line. From this, we conclude that the light ray which falls perpendicular to one side of the slab surface comes out without any deviation.
- Now, draw a line, from the point of intersection where side AB of the rectangle and perpendicular meet in such a way that it makes 30° angle with normal.
- This line represents the incident ray falling on the slab and the angle it makes with the normal represents the angle of incidence.
- Now place the slab on the paper in such a way that it fits in the rectangle drawn.
- Fix two identified pins on the line making a 30° angle normal such that they stand vertically with equal height.
- By looking at the two pins from the other side of the slab, fix two pins in such a way that all pins appear to be along a straight line.
- Remove the slab take out the pins, and draw a straight line by joining the dots formed by the pins up to the edge ‘CD’ of the rectangle.
- Draw a perpendicular on to the line ‘CD’ and measure the angle between the emergent ray and the normal. This is called the angle of emergence.
- We will notice that the incident and emergent rays are parallel and the distance between the parallel rays is called lateral shift.
(OR)
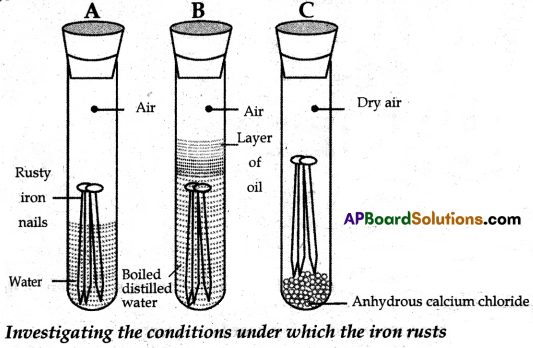
(B) (i) Take three test tubes and place clean iron nails in each of them.
(ii) Label these test tubes A, B, and C. Pour some water into test tube A and cork it.
(iii) Pour boiled distilled water into test tube B, add about 1 ml of oil, and cork it. The oil will float on water and prevent the air from dissolving in the water.
(iv) Put some anhydrous calcium chloride in test tube ‘C’ and cork it. Anhydrous calcium chloride will absorb the moisture, if any from the air.
(v) Leave these test tubes for a few days and then observe them.
(vi) You will observe that iron nails, in test tube ‘A’ get rusted. But they do not get rusted in test tubes ‘B’ and ‘C’.
(vii) In test tube ‘A’, the nails are exposed to air and wafer. In test tube ‘B’ the nails are exposed to only water and the nails in test tube ‘C’ are exposed to dry air.
(viii) From this activity we conclude that both air and water are necessary for corrosion (Rusting) of iron.