These TS 8th Class Physical Science Important Questions 9th Lesson Electrical Conductivity of Liquids will help the students to improve their time and approach.
TS 8th Class Physical Science Important Questions 9th Lesson Electrical Conductivity of Liquids
Question 1.
Do you know the reason behind getting electric shock while working with wet hands?
Answer:
We get electric shock while working with wet hands because tap water is a good conductor of electricity. If our hands are wet while working with a live electric circuit then the circuit gives electric shock.
Question 2.
How the does the electric current flow through water?
Answer:
The water that we get from sources such as taps, hand pumps, wells, ponds is not pure but a solution. Small amounts of mineral salts are naturally present in it. These mineral salts convert water into a conductor. This water is thus a conductor of electricity. On the other hand, distilled water is free of salts, and thus a poor conductor of electricity.
Question 3.
Why do we use LED in the tester Instead of a bulb?
Answer:
LED glows even when a very weak current is passing through the circuit. Thus, it helps in testing flow of electricity in conductions when meager current is passing through the circuit.
Electric cell
Question 4.
Can you produce electric current in another ways apart from dry cells?
Answer:
yes, I can produce electric current by using 4 lemons, 4 copper plates, and 4 paper clips. By connecting these 4 Lemons with electrodes, I produce current enough to glow a LED.
![]()
Question 5.
Do you know, how was the first cell made?
Answer:
In 1800, Alessandro Volta of Italy made his first cell in 1800 using zinc and copper plates dipped in sulphuric acid. He found that it did not require an animal’5 body to generate electricity. It is possible to generate electricity if two different metals are placed in some liquids. His discovery made him famous in the realm of science.
Question 6.
What are the precautions to be taken during the process of electroplating?
Answer:
- The object to be electroplated should be free from greasy mailer.
- The surface of the article should be rough so that the metal deposited sticks permanently.
- The concentration of the electrolyte should be so adjusted as to get smooth coating.
- Current must be the same throughout.
Question 7.
A child staying in a coastal region tests the drinking water and also the seawater with his tester. He finds that the compass needle deflects more in the case of seawater. Can you explain the reason?
Answer:
Sea water contains higher amounts of salts in comparison to drinking water, hence sea water is a better conductor of electricity. This is the reason that the compass needle deflects more in case of seawater.
Question 8.
Is It safe for the electrician to carry out electrical repairs outdoors during heavy downpours? Explain.
Answer:
It is never safe for the electrician to carry out electrical repairs during heavy downpour. Because during heavy downpours there is a high risk of electrocution.
Question 9.
Prepare a list of objects around you that are electroplated.
Answer:
Ornaments, wheel rims of vehicles, handlebars of cycle and motorcycles, bath taps, kitchen gas burner, bottom of cooking utensils, handles of doors, tin cans etc.
![]()
Question 10.
What is the difference between electrolytic cell and galvanic cell?
Answer:
Electrolytic cell: A device in which electrical energy is converted into chemical energy.
Gahanic or electrochemical cell: A device in which chemical energy is converted into electrical energy.
Question 11.
Air and water are bad conductors of electricity. Then how the electric charge from clouds reaches the earth?
Answer:
During thunderstorms, some amazing things occur. Thunder and lightning provides extraordinary air behaviour. Cumulonimbus clouds possess a strong electrical charge. The charge on the surface of the cloud is more positive. These positive ions begin to move towards the base which is strongly negative.
Because air is a very poor conductor of electricity, these ions can not easily travel to one another. Lightning is the result of this electrical field building upto such an extent to overcome the poor conductivity of the air. Suddenly the insulating capacity of the air breaks down and lightning occurs.
Question 12.
Write the uses of electroplating.
Answer:
1. Electroplating is widely used in industry for coating metal objects with a thin layer of different metals.
Eg :
- Metals like iron which are easily corroded are coated with deposits of nickel or chromium which are most resistant by electroplating method.
- In repairing worn-out parts of machinery, suitable metal is deposited on the affected parts of the machinery by electroplating method.
2. Electroplating is also done for ornamentation and decoration purposes. Several articles made of copper or its alloys, such as jewellery tablewares, decoration pieces are coated with silver or gold.
3. Processed food items are preserved in tin-coated iron cans. Tin is less reactive to the food than iron.
4. Zinc-coated iron is used for bridges and in automobiles. Zinc-coated iron is more resistive to corrosion and formation of rust.
![]()
Question 13.
In case of a fire, before the fire men use the water, they shut off the main electrical supply for the area. Explain why they do this.
Answer:
The water used in water hoses by the firemen is not pure water and it conducts electricity. Firemen shut off the main electrical supply for the area because, if the supply of electricity continues, it may be high risk of electrocution of the whole area due to water.
Question 14.
We get some items made from iron wire in which Iron wire is coated with plastic. Is plastic coated by the process of electroplating? Why plastic cannot be coated on a metal by the process of electroplating?
Answer:
In the process of electroplating metal ions in an electrolyte solution are deposited on a cathode. In this process,
anode is generally made up of metal being plated, but plastic is a nonmetal and poor conductor of electricity. Hence plastic can not be coated on a metal by the process of electroplating’. This could be done by the ‘non-metallic coating process such as ‘wire and cable coating’.
Question 15.
Refer to the Activity 3 in the chapter. Start with distilled water. The LED would not glow. Add two drops of some acid to distilled water and check the glow of LED. Add two more drops and check the intensity of the glow. Repeat the activity 5 to 6 times by adding 2 drops of the same acid each time. Do you see any difference in the intensity of glow with increasing acid content of water? What can be inferred from the above observations? Repeat the entire activity by taking a solution of baking soda and adding
drops of it to distilled water instead of acid. Write differences and similarities.
Answer:
- With the reference of Activity -3 (transforming a poor electric conductor into a good conductor). By starting with distilled water, the LED would not glow, since distilled water is a poor conductor of electricity.
- By adding 2 drops of acid to distilled water, then we observe, the LED glows.
- By adding 2 more drops of acid to distilled water, then the LED glows hut relatively bright.
- By repeating this activity 5 to 6 times by adding 2 drops of the same acid, each time we observe the change of glow of LED. The brightness of LED glow increases with the addition of acid in each time.
- Inference 1: The conductivity of distilled water increases with increase of concentration of acid.
- By repeating the entire activity by taking a solution of baking soda instead of acid, we observe the same result. Since baking soda is a base.
- Inference 2: The conductivity of distilled water increases with increase of concentration of base.
- Similarity: Result is same in both cases cl adding acid or base,
- Difference: First we took acid, later base (baking soda).
![]()
Question 16.
How do you appreciate the efforts of Luigi Galvani and Alessandro Volta In discovering a cell and making a stored electric energy available to human beings?
Answer:
Luigi Galvani discovered something he named ‘animal electricity when two different metals were connected in series with the frog’s leg and to one another. Volta realised that the frog’s leg served as both a conductor of electricity and as a detector of electricity.
Volta repeated his experiments using different Liquids instead of frog’s legs. These experiments showed the way to discover a source of electricity. Volta made his first cell in ‘1800. His discovery made him famous in the realm of science. Now – a – days we can not imagine life without a cell. Most of electric and electronic appliances like calculator, cell phone, laptop, radio, camera, different types of toys etc essentially consists of electric cells.
diagrams
1.
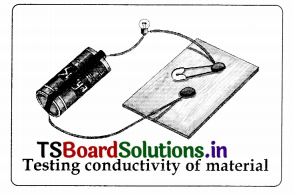
2.
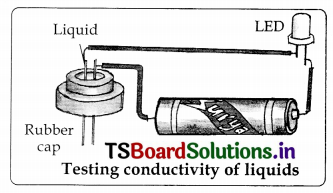
3.
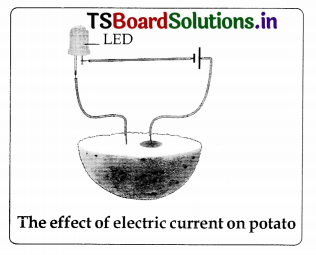
4.
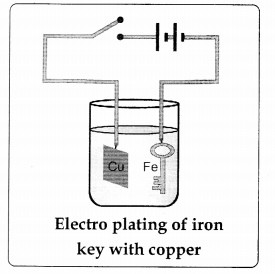
5.
Multiple choice questions
Question 1.
Plastic is …………………….. . ( )
A) a conductor
B) an insulator
C) a semiconductor
D) a metal
Answer:
B) an insulator
Question 2.
Which of the following is not an insulator? ( )
A) plastic
B) glass
C) steel
D) cotton
Answer:
C) steel
Question 3.
The other name of electrolytic cell ( )
A) ammeter
B) electrode
C) voltmeter
D) voltameter
Answer:
C) voltmeter
![]()
Question 4.
The process is to be done to prevent corrosion of iron objects ( )
A) electrolysis
B) electroplating
C) refining
D) metallurgy
Answer:
B) electroplating
Question 5.
A positively charged ion is called ( )
A) cation
B) anion
C) atom
D) neutron
Answer:
A) cation
Question 6.
An instrument used to measure the current flow in a circuit is
A) voltmeter
B) ammeter
C) voltameter
D) cell
Answer:
B) ammeter
Question 7.
Which is not a non-electrolyte? ( )
A) sugar solution
B) urea
C) sodium chloride solution
D) distilled water
Answer:
C) sodium chloride solution