Students get through AP Inter 1st Year Chemistry Important Questions 5th Lesson Stoichiometry which are most likely to be asked in the exam.
AP Inter 1st Year Chemistry Important Questions 5th Lesson Stoichiometry
Very Short Answer Questions
Question 1.
How many number of moles of glucose are present in 540 grams of glucose. [Imp.Q][IPE’ 14]
Answer:
Weight of glucose = 540 g
Molecular wt. of glucose (C6H12O6) = 180

Question 2.
Calculate the weight of 0.1 mole of Sodium carbonate. [TS 19, 20][AP 16]
Answer:
No. of moles of Na2CO3 (n) = 0.1,
G.M.W of Na2CO3 = 106
Weight of Na2CO3 = n × GMW
= 0.1 × 106 = 10.6 g
Question 3.
How many molecules of glucose are present in 5.23 g of glucose ( Molecular weight of glucose 180 g) [Imp.Q]
Answer:
Weight of glucose (W) = 5. 23 g
GMW of glucose = 180 g
Avogadro’s number (N0) = 6.023 × 1023

Question 4.
Calculate the number of molecules present in 1.12 × 10-7 c.c of a gas at STP (c.c – cubic centimeter = cm³)
Answer:
Volume of gas (V) = 1.12 × 10-7 cc
Avogadro’s number (N0) = 6.023 × 1023
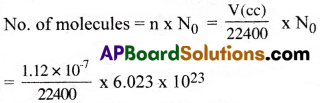
= 3.011 × 1012 molecules.
Question 5.
The empirical formula of a compound is CH2O.Its molecular weight is 90. Calculate the molecular formula of the compound. [TS 22][AP 16]
Answer:
Empirical formula (CH2O) weight = 12 + 2 + 16 = 30
Molecular weight = 90
![]()
Molecular formula = (Empirical formula)n
= (CH2O)3 = C3H6O3
![]()
Question 6.
Balance the following equation by the oxidation number method
Cr(S) +Pb(NO3)2(aq) → Cr(NO3)3(aq) + Pb(s)
Answer:
1) Writing the skeleton equation
Cr + Pb(NO3)2 → Cr(NO3)3 + Pb
2) Writing the oxidation number of every atom above its symbol.
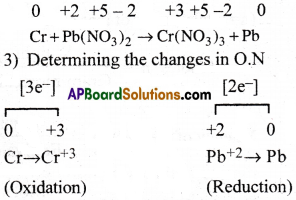
4) Cris-crossing the changes in O.N
2Cr + 3Pb(NO3)2 → Cr(NO3)3 + Pb
5) Balance the atoms other than ‘H’ and ‘O’
2Cr + 3Pb(NO3)2 → 2Cr(NO3)3 + 3Pb
It is balanced equation.
Question 7.
What volume of H2 at STP is required to reduce 0.795 g of CuO to give. Cu and H2O [Imp.Q]
Answer:
The balanced equation is

1 mole CuO : 63.6+ 16 = 79.6 g
1 mole H2 at STP = 22.4 lit.
Volume of H2 required = \(\frac{0.795}{79.6}\) × 22.4
= 0.224 lit or 224 ml.
∴ Volume of H2 at STP required to reduce 0.795 g of CuO is 224ml.
Question 8.
Calculate the volume of O2 at STP required to completely burn 100ml of acetylene. [Imp.Q]
Answer:
The balanced equation is
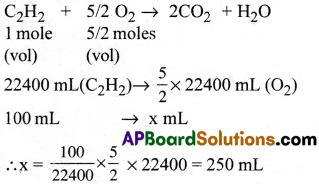
∴ Required volume of 02 is 250 mL.
Question 9.
Now-a-days it is thought that oxidation is simply decrease in electron density and reduction is increase in electron density. How would you justify this?
Answer:
Yes, it is true.
Explanation:
a) In oxidation there will be loss of electrons That means number of electrons decrease in an atom.
∴ Electron density decreases.
b) In reduction there will be gain of electrons That means number of electrons increase in an atom.
∴ Electron density increases.
Question 10.
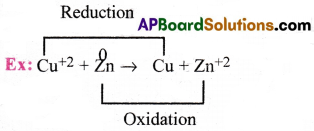
What is a redox concept? Give an example. [Imp.Q]
Answer:
Redox reactions are the reactions in which both reduction and oxidation takes place simultaneously.
Question 11.
Calculate the muss percent of the different elements present in sodium sulphate (Na2SO4).
Answer:
Given compound is sodium sulphate (Na2SO4).
Molecular weight of the compound = 2 (23) + 1 (32) +4(16) = 46 +32 + 64 = 142
Step -I
Mass percent of ’Na’
142 gms of Na2SO4 → 46gms of Na
100 gms of Na2SO4 → x gm
∴ x = \(\frac{100\times46}{142}\) = 32.9%
Step-II
Mass percent of ‘S’
142 gms of Na2SO4 → 32gms of S
100 gms 0f Na2SO4 → x gm
∴ x = \(\frac{100\times32}{142}\) = 22.53%
Step -III:
Mass percent of ‘O’
142 gms of Na2SO4 → 64 gms of Oxygen
100 gms of Na2SO4 → ?x
∴ Mass percentx = \(\frac{100\times64}{142}\) = 45.07%
∴ Mass percents of Na, S, O are 32.39, 22.53, 45.07.
![]()
Question 12.
What do you mean by significant figures?
Answer:
Significant figures are meaningful digits which are known with certainty.
The uncertainity in the experimental or calculated values is indicated by mentioning the number of significant figures.
Question 13.
If the speed of light is 3.0 × 108 ms-1, calculate the distance covered by light in 2.00 ns.
Answer:
Speed of light = 3.0 × 108 ms-1
∴ Distance covered by light in 1 sec
= 3.0 × 108m
Distance covered by light in 2.00 ns
= 2.00 × 10-9 s
is 2.00 × 10-9 × 3.0 × 108 = 0.6 m
Short Answer Questions
Question 1.
The approximate production of Sodium carbonate per month is 424 × 106 g. While that of methyl alcohol is 320 × 106 gm. Which is produced more in terms of moles?
Answer:
Moles of Sodium carbonate produced per month = \(\frac{424\times10^6}{106}\) = 4 × 106
Moles of Methyl alcohol produced per month = \(\frac{320\times10^6}{32}\) = 107
So Methyl alcohol produced in terms of moles is more.
Question 2.
How much minimum volume of CO at STP is needed to react completely with 0.112 L of O2 at 1.5 atm and 127°C to give CO2
Answer:
Reaction between CO and O2.
2CO + O2 → 2CO2
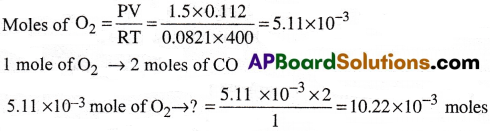
∴ The minimum volume of CO required at STP = 10.22 × 10-3 × 22400 = 229.32 ml.
Question 3.
Chemical analysis of a carbon compound gave the following percentage composition by weight of the elements present, carbon = 10.06% , hydrogen = 0.84 % , chlorine = 89.10% . Calculate the empirical formula of the compound.
Answer:
Step I : Percentage composition of the elements present in the compound.
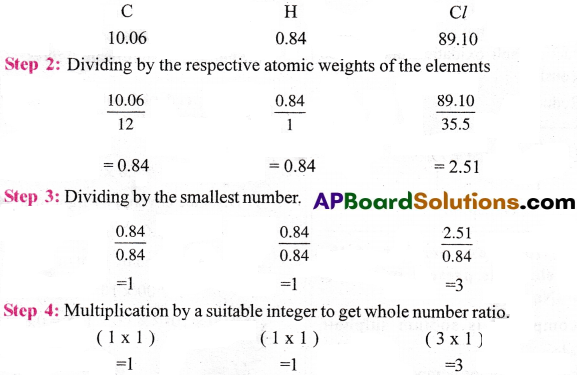
∴ The empirical formula of the compound is C1H1Cl3 = CHCl3.
Question 4.
A carbon compound on analysis gave the following percentage composition, Carbon 14.5%, Hydrogen 1.8%, Chlorine 64.46%, Oxygen 19.24%. Calculate the empirical formula of the compound.
Answer:
Step 1 : Percentage composition of the elements present in the compound.(%)
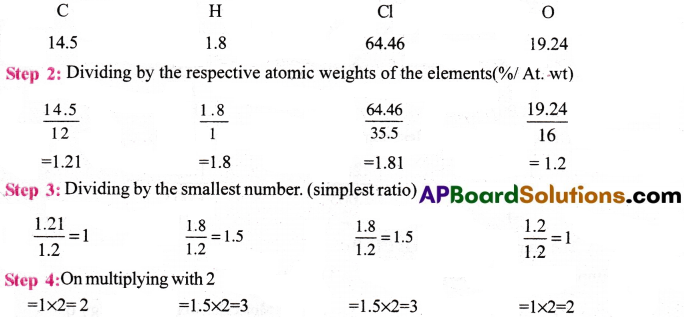
∴ The empirical formula of the compound = C2H3Cl3O2
![]()
Question 5.
Calculate the empirical formula of a compound having percentage composition :
potassium (K) = 26.57, Chromium (Cr) = 35.36, Oxygen (O) = 38.07.
(Given the atomic weights of K, Cr and O as 39 ; 52 and 16 respectively)
Answer:
Step 1 : Percentage composition of the elements present in the compound.
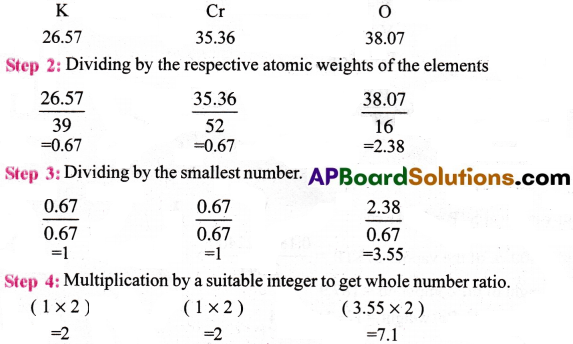
∴ The empirical formula of the compound K2Cr2O7
Question 6.
A carbon compound contains 12.8% carbon, 2.1% hydrogen, 85.1% bromine. The molecular weight of the compound is 187.9. Calculate the molecular formula. [TS 20][AP 17, 19, 22]
Answer:
Step 1: Percentage composition of the elements present in the compound(%).
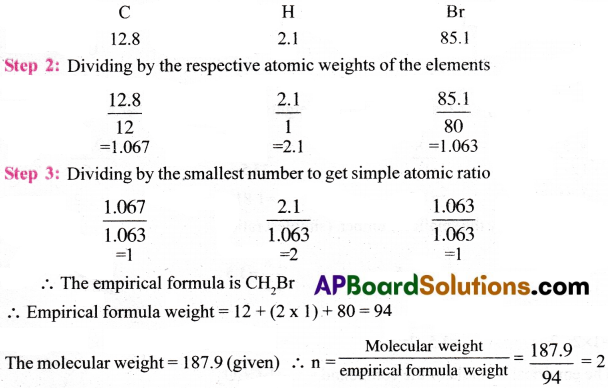
Molecular formula = (Empirical formula)n = (CH2Br)2 = C2H4Br2
Question 7.
0.188 g of an organic compound having an empirical formula CH2Br displaced 24.2 cc. of air at 14°C and 752 mm pressure. Calculate the molecular formula of the compound. (Aqueous tension at 14°C is 12 mm)
Answer:
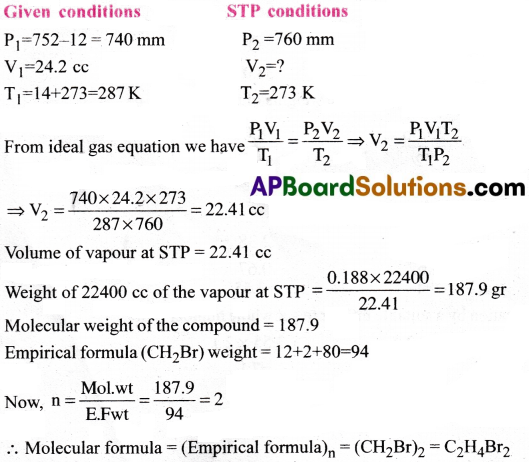
Question 8.
Calculate the amount of 90% of H2SO4 required for the preparation of 420 kg HCl.
2NaCl + H2SO4 → Na2SO4 + 2HCl
Answer:
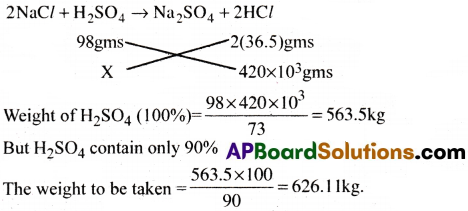
Question 9.
An astronaut receives the energy required in his body by the combustion of 34g of sucrose per hour. How much oxygen he has to carry along with him for his energy requirement in a day?
Answer:
Wt. of sucrose required per day = 34 × 24 = 816 g.
Moles of sucrose = \(\frac{W}{M.Wt}=\frac{816}{342}\) = 2.385
Sucrose react with oxygen as follows.
C12H22O11 + 12O2 → 12CO2 + 11H2O
According to the above reaction
1 mole sucrose requires 12 moles of O2
2.385 moles requires = \(\frac{2.355\times12}{1}\) = 28.63
Wt. of oxygen = No. of moles × Mol. Wt. = 28.63 × 32 = 916.2 g.
Question 10.
What volume of CO2 is obtained at STP by heating 4 g of CaCO3?
Answer:
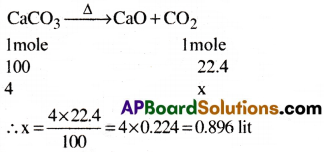
Question 11.
When 50 gm of a sample of sulphur was burnt in air 4% of the sample was left over. Calculate the volume of air required at STP containing 21% oxygen by volume.
Answer:
Amount of Sulphur taken = 50g Wt. of sulphur left = 4% = 2g
Wt. of sulphur reacted = 50 – 2 = 48 g
Sulphur burns in air according to the reaction. S + O2 → SO2
Moles of Sulphur = \(\frac{48}{32}\) = 1.5 Moles of Oxygen required = 1.5
Volume of Oxygen at STP = 22.4 × 1.5 = 33.6 lit.
Volume of air = \(\frac{33.6\times100}{21}\) = 160lit. (∴ air is 21% O2)
![]()
Question 12.
Calculate the volume of oxygen gas required at STP conditions for the complete combustion of 10cc of methane gas at 20°C and 770 mm pressure.
Answer:
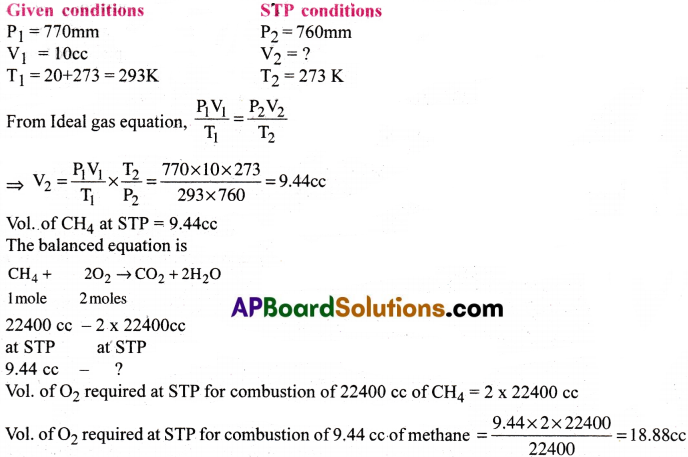
Question 13.
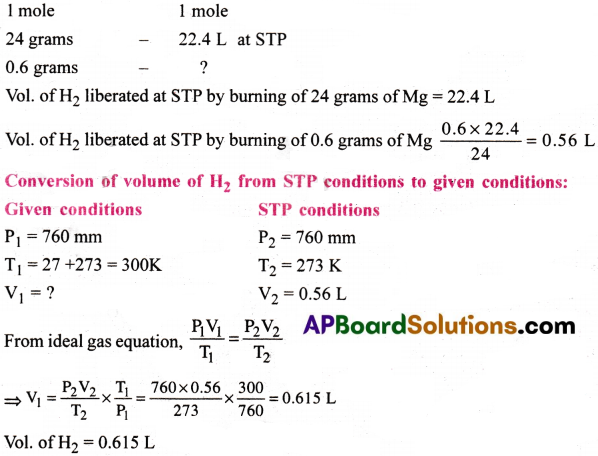
Calculate the volume of H2 liberated at 27°C and 760 mm of Hg pressure by action by 0.6g magnesium with excess of dil HCl.
Answer:
Mg + 2HCl → MgCl2 + H2
Question 14.
Explain the role of redox reactions in titrimetre processes and galvanic cells.
Answer:
Role of redox reactions in titrimetric quantitative analysis:
The process of adding a standard solution to the titrand till the reaction is just complete is called titration. The point at which the titrand just completely reacts with the standard solution is called “equivalence point” or “end point”. During titration, redox reaction takes place and hence these titration are known as Redox titrations.
In redox titration the completion of the titration is detected by a suitable method like
(a) observing a physical change.
Ex: The light pink clour of KMnO4 titrations. [KMnO4 is a self indicator]
(b) by using a reagent known as indicator which gives a clear visual change in its colour.
Ex (1) In Cr2O7-2 (dichromate) titrations, diphenyl amine is used as reagent and at the end point it produces intense blue colour due to its oxidation by Cr2O7-2
Ex (2) In the titration of Cu+2 with I– (Iodometry)
2Cu+2(aq) + 4I–(aq) → Cu2I2(s) + I2(aq)
The I2 formed in the redox reaction gives a deep blue colour with starch solution., added to the flask.
In this way redox reactions are taken as the basis for titrimetric analysis with MnO–7, Cr2O7-2 etc. as oxidising agents and S2O-23 etc as reducing agents.
Role of Redox reactions in galvanic cells:
If a zinc rod kept in copper sulphate solution the following redox reaction takes place.
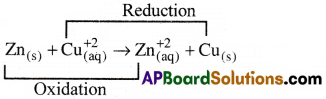
In this redox reaction the transfer of electrons from Zn(s) to Cu+2(aq) takes place directly. The same transfer of electrons can also be done indirectly in a galvanic cell (Daniel cell).
Cells in which chemical energy is converted into electrical energy are called galvanic cells. Daniel cell is a best example for a galvanic cell. The Daniel cell consists of two beakers containing zinc rod dipped in ZnSO4(aq) solution in one beaker and a copper rod dipped in CuSO4(aq)solution in a second beaker. The two beakers are connected by an inverted U-tube , known as salt bridge. The two rods are connected by means of wires to the terminals of an ammeter. Redox reaction takes place in each of the beakers. Each beaker contains both oxidised and reduced forms of the respective species. The two types of species present together in each beaker is called a redox couple. Each beaker contains a redox couple. The oxidised and reduced forms are separated by a vertical line or a slash
Ex: Zn(s)/Zn+2(aq)
In the above arrangement the two redox couples are represented by Zn+2/Zn and Cu+2/Cu. As the metal is in two different, oxidation states at the interface (say Zn/Zn+2), some potential is developed, which is called ‘electrode potential’. These electrode potentials are very useful in metallurgy, electroplating etc.
In this way redox reactions play an important role in galvanic cells.
![]()
Question 15.
Define and explain molar mass.
Answer:
Molar mass :
The mass of one mole of any substance in grams is called its molar mass. .
Ex: Molar mass of sulphuric acid = H2SO4 = 2(1) + 1(32) + 4(16) = 2 + 32 + 64 = 98gm.
Thus molar masses are atomic weights, molecular weights, formula weights etc. expressed in grams.
Gram atomic weight is atomic weight expressed in grams. Gram molecular weight is molecular weight expressed in grams.
Gram atom :
One gram atomic weight of a substance is known as gram atom.
Gram molecule :
One gram molecular weight of a substance is known as gram molecule.
Mole:
It is the mass of a substance which contains Avogadro number of structural units.
1 mole = 1 gram molecule = 1 gram molecular weight = Mass of 6.023 × 1023 molecules in grams.
1 mole = 1 gram atom = 1 gram atomic weight = Mass of 6.023 × 1023 atoms in grams.
Question 16.
Determine the empirical formula of an oxide of iron which has 69.9% iron and 30.1% dioxygen by mass.
Answer:
Step 1 : Percentage composition of the elements present in the compound.
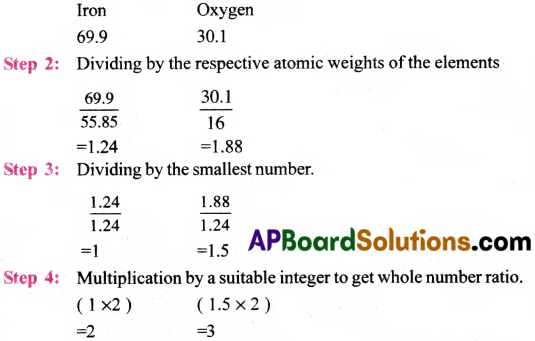
∴ Empirical formula of oxide of iron = Fe2C3
Question 17.
What are disproportionation reactions? Give an example. [Mar’ 10][TS 15, 16, 22]
Answer:
Disproportionation reactions :
The redox reactions, in which the same element undergoes both oxidation & reduction are called disproportionation reactions.

Here Cl2 undergoes both oxidation and reduction reactions.
Question 18.
What are comproportionation reactions? Give an example.
Answer:
Comproportionation reactions : It is the reverse of disproportonation.
In this reaction, an element in a higher oxidation state reacts with the same element, in a lower oxidation state to give the element in an intermediate oxidation state.
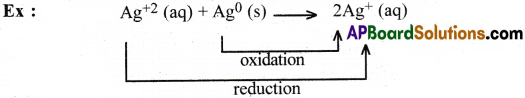
Question 19.
Calculate the mass of Sodium acetate (CH3COONa) required to make 500mk of 0.375 molar aqueous solution. Molar mass of Sodium acetate is 82.0245 gmol-1.
Answer:
Molarity = 0.375M, V= 500ml, GMW = 82.0245, w =?

The mass of CH3COONa present in 500 ml = 15.38 gm.
Question 20.
What is the concentration of sugar (C11H22O11) in mol L-1 if 20g are dissolved in enough water to make a final volume upto 2L?
Answer:
w = 20gm
GMW of sucrose = 342g
V = 2L
![]()
Question 21.
How many significant figures are present in the following?
(i) 0.0025 (ii) 208 (iii) 5005 (iv) 126, 000 (v) 500.0, (vi) 2.0034
Answer:
i) 0.0025 has two significant figures.
ii) 208 has three significant figures.
iii) 5005 has four significant figures.
iv) 126,000 has three significant figures.
v) 500.0 has four significant figures.
vi) 2.0034 has five significant figures.
![]()
Question 22.
Round up the following upto three significant figures:
(i) 34.216 (ii) 10.4107 (iii) 0.04597 (iv) 2808
Answer:
i) 34.216 is rounded off to 34.2
ii) 10.4107 is rounded off to 10.4
iii) 0.04597 is rounded off to 0.0460
iv) 2808 is rounded off to 2.81 × 103
Question 23.
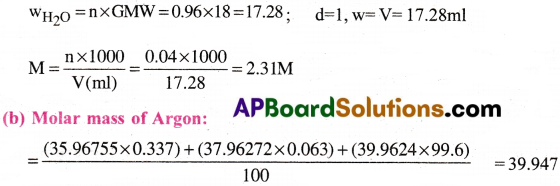
a) Calculate the molarity of a solution of ethanol in water in which the mole fraction of ethanol is 0.040 (assume the density of water to be one), b) use the data given in the following table to calculate the molar mass of naturally occurring argon isotopes:
| Isotope | Isotopic molar mass | Abundance |
| 36Ar | 35.96755 gmol-1 | 0.337% |
| 38Ar | 37.96272 gmol-1 | 0.063% |
| 40Ar | 39.9624 g mol-1 | 99.600% |
Answer:
a) Mole fraction of Ethanol = 0.04
Sum of the molefraction = 1
Mole fraction of Ethanol + Mole fraction of Water = 1
0.04 + Mole fraction of Water = 1 ⇒ Mole fraction of Water = 1 – 0.04 = 0.96
Question 24.
A welding fuel gas contains carbon and hydrogen only. Burning a small sample of it in oxygen gives 3.38 g Carbondioxide, 0.690 g of water and no other products. A volume of 10.0 L (measured at STP) of this welding gas is found to weigh 11.6g. Calculate (i) empirical formula, (ii) molar mass of the gas, and (iii) molecular formula.
Answer:
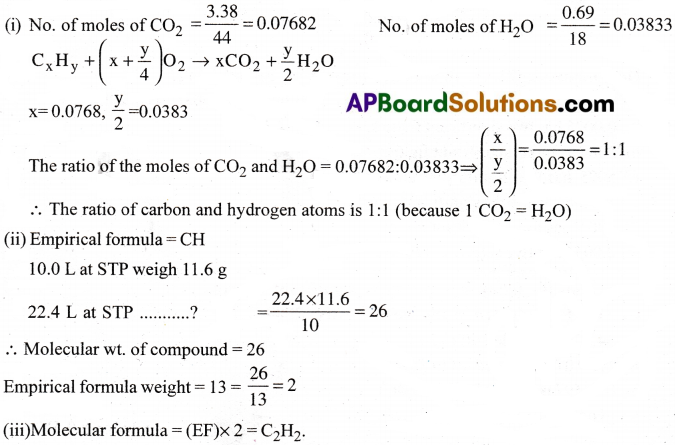
Question 25.
Calcium Carbonate reacts with aqueous HCI to give CaCI2 and C02 according to the reaction, CaCO3(s) + 2HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l). What mass of CaCO3 is required to react completely with 25 mL of 0.75 M HCl?
Answer:
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l)
Moles of HCl = \(\frac{25\times0.75}{1000}\) = 0.01875
With 2 mol. of HCl, 1 mole of CaCO3 reacts.
With 0.01875 mol. of HCl, the mole of CaCO3 that reacts = \(\frac{0.01875\times1}{2}\) = 0.009375
∴ Wt. of CaCO3 = 0.009375 × 100 = 0.9375g.
Question 26.
Chlorine is prepared in the laboratory by treating Manganese dioxide (MnO2) with aqueous hydrochloric acid according to the reaction 4HCl(aq) + MnO2(s) → 2H2O(l) + MnCl2(aq) + Cl2(g)
How many grams of HCI react with 5.0 g of manganese dioxide?
Answer:
4HCl + MnO2 → Cl2 +MnCl2
1 mole MnO2 reacts with 4 moles HCl
i.e., 87gms of MnQ2 ——- 4 × 36.5g
5gmsofMnO2 ——– of HCl
∴ x = \(\frac{4\times5\times36.5}{87}\) = 8.3g
![]()
Question 27.
To 50ml of 0.1N Na2CO3 solution 150ml of H2O is added. Then calculate a normality of resultant solution.
Answer:
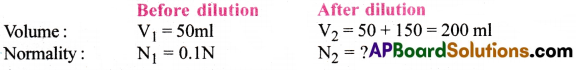
According to dilution law, V1N1 = V2N2 50 × 0.1 = 200 × N2 N2 = \(\frac{50\times0.1}{200}\) = 0.025N
Normality of resultant solution = 0.025N
Question 28.
Calculate the volume of 0.1N H2SO4 required to neutralize 200ml of 0.2N of NaOH solution. It is an acid base neutralization reaction. Hence, at the neutralization point, Number of equivalents of acid — Number of equivalents of base.
Answer:

Question 29.
Calculate normality of H2SO4 solutions if 50ml of it completely neutralizes 250ml of 0.1N Ba(OH)2 solution.
Answer:
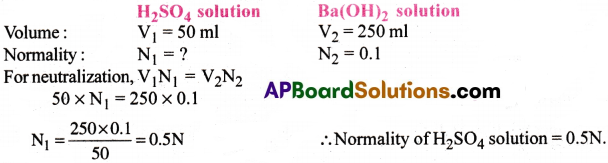
Question 30.
Calculate normality of H2SO4 solution. If 50ml of it completely neutralized 250ml of 0.2N sodium hydroxide Na(OH) solution.
Answer:
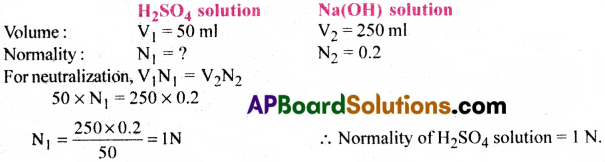
Question 31.
Calculate the volume of 0.1 M KMnO4 required to react with 100ml of 0.1M H2C2O4.2H2O solution in presence of H2SO4.
Answer:
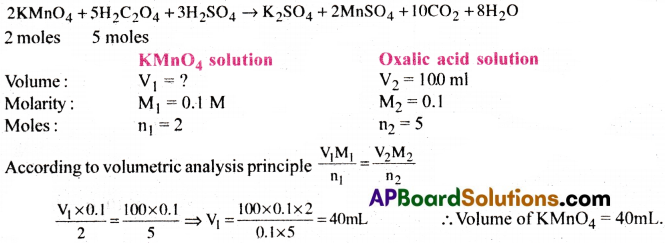
Question 32.
Assign Oxidation number to the underlined elements in each of the following species:
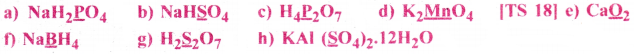
Answer:
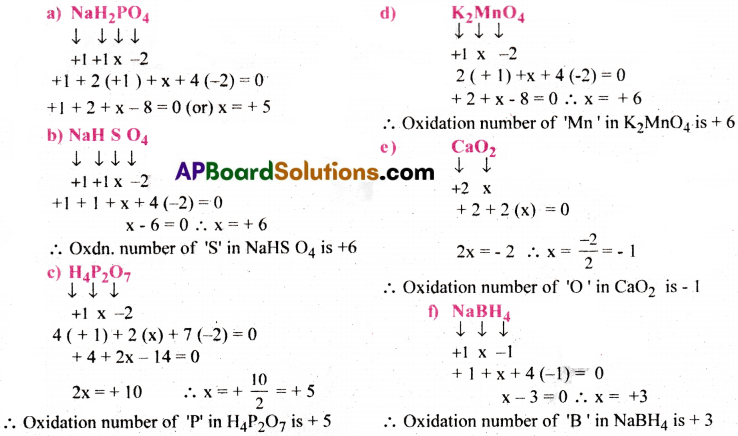
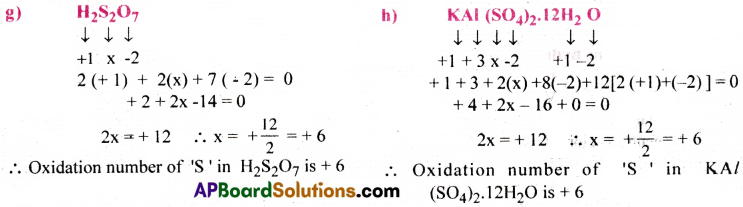
Question 33.
What are the oxidation number to the underlined elements in each of the following and how do you rationalise your results.
a) Fe3O4 b) H2S4O6 c) KI3 d) KMnO4
Answer:
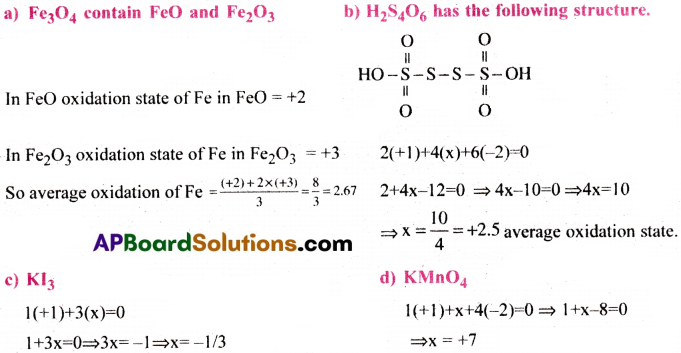
Question 34.
Justify that the following reactions are redox reactions.
a) CuO(s) + H2(g) → Cu(s) + H2O(g)
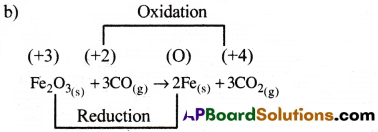
b) Fe2O3(s) + 3CO(g) → 2Fe(s) + 3CO2(g)
c) 4BCl3(g) + 3LiAlH4(s) → 2B2H6(g) + 3LiCl(s) + 3AlCl3(s)
d) 2K(S) + F2(g) → 2K+F–(s)
e) 4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g)
Answer:
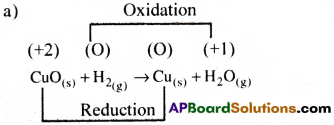
In this reaction both oxidation and reduction takes place. So, it a redox reaction.
In this reaction both oxidation and reduction takes place. So, it is a redox reaction.
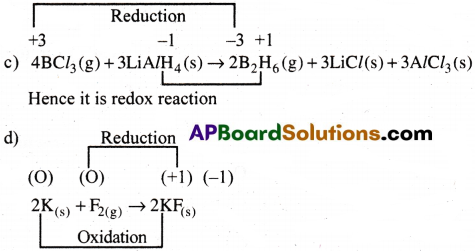
In this reaction both oxidation and reduction takes place. So, it is a redox reaction.
e) 4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g)
The oxidation of N increases from -3 to +2 in the conversation of NH3 to NO. It is oxidation.
The oxidation number of O2 changes from zero to -2.
It is reduction. So it is redox reaction.
![]()
Question 35.
Fluorine reacts with ice and results in the change. H2O(s) + F2(g) → HF(g) + HOF(g) Justify that this reaction is a redox reaction.
Answer:
Let us write the oxidation number of each atom involved in the reaction.
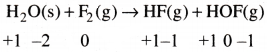
Oxidation number of ‘O’ increases from -2 to 0, and oxidation number of’F’ decreases from 0 to -1. thus the above reaction is a Redox reaction.
Question 36.
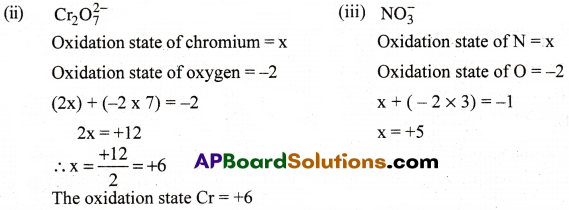
Calculate the oxidation number of sulphur, chromium and nitrogen ion H2SO5, Cr2O2-7 and NO–3 Suggest structure of those compounds.
Answer:

The compound contains one Peroxy (O-O) linkage.
∴ 2(+1) + 1(x) + 3(-2) + 2(-1) = 0
2 + x – 6 – 2 = 0 x – 6 = 0 x = +6
Question 37.
Write the formulae for the following compounds.
a) Mercury (II) chloride
b) Nickel (II) sulphate
c) Tin (IV) oxide
d) Thallium (I) chloride
e) Iron (III) sulphate
f) Chromium (III) oxide.
Answer:
a) HgCl2
b) NiSO4
c) SnO2
d) TlCl
e) Fe2(SO4)3
f) Cr2O3
Question 38.
Suggest a list of the substance where carbon exhibit oxidation sates from -4 to +4 and nitrogen from – 3 to +5.
Answer:
List of carbon compounds that exhibit oxidation states from – 4 to +4.
The underlined carbon in the following compounds have the oxidation sate mentioned.
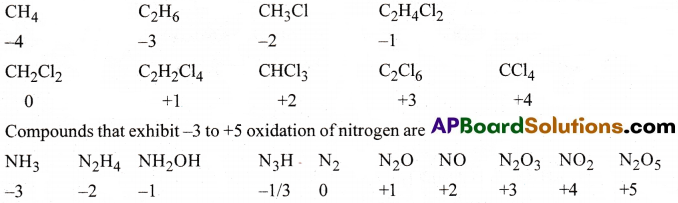
Question 39.
While sulphur dioxide and hydrogen peroxide can act as oxidising as well as reducing agents in their reactions, ozone and nitric acid act only as oxidants. Why?
Answer:
In sulphur dioxide sulphur is in +4 oxidation state. It can increase its oxidation number up to +6 while acting as reducing agent and can decrease its oxidation number up to either 0 or -2. While act as oxidising agent.
Similarly in hydrogen peroxide oxidation number of oxygen is -1. It can increase its oxidation number upto zero and can decrease its oxidation number to -2.
Therefore SO2 and H2O2 can act as oxidising and reducing agents in their reactions.
In ozone the oxidation number of oxygen is zero. It can only decrease its oxidation number but cannot increase its oxidation number. This is because it is only the most electronegative atom next to fluorine.
In nitric acid oxidation state of nitrogen is +5. It cannot increase its oxidation state because it is the maximum oxidation state of nitrate. It can only decrease its oxidation number.
Because of these reasons ozone and nitric acid can act only as oxidising agents.
Question 40.
Consider the reactions.
a) 6CO2 (g) + 6H2O(l) → C6H12O6 (aq) + 6O2(g)
b) O3 (g) + H2O2 (1) → H2O(1) + 2O2(g)
Why it is more appropriate to write these reactions as
a) 6CO2(g) + 12H2O(1) → C6H12O6 + 6H2O(1) + 6O2(g)
b) O3(g) + H2O2 (l) → H2O(l) + O2(g) + O2(g)
Also suggest a technique to investigate the path of the above (a) and (b) redox reactions.
Answer:
Plants absorb carbon dioxide from air, water from soil and convert them into carbohydrates in the presence of sunlight and Chlorophyll. This process is known as photosynthesis.
During photosynthesis plants liberate oxygen. The oxygen will be liberated from water but not form carbondioxide. The following reaction cannot explain the liberation of oxygen from water because in this reaction from 6H20 molecules only 302 can be liberated.
a) 6CO2 (g) + 6H2O(l) → C6H12O6(aq) + 6O2(g)
But the following reaction can explain the liberation 602 molecules from water.
6CO2(g) + 12H2O(l) → C6H12O6(aq) + 6H2O(l) + 6O2(g)
The path of the reaction can be traced by taking labile 18O in H2O. The liberated oxygen contain the total labile 18O which indicates the oxygen is liberated from water.
6CO2(g) + 12H2O(l) → C6H12O6(aq) + 6H2O(l) + 6O2(g)
b) The reaction between O3 and H2O2 can be written as follows:

So it is appropriate to the equation as above instead of
O3 + H2O2 → H2O + 2O2
In the reaction O3 + H2O2 → H2O + O2 + O2
One of the O2 liberated from O3 and the another from H2O2. This can be traced by using 18O isotope in H2O2.
![]()
Question 41.
The compound AgF2 is unstable compound. However, if formed, the compound acts as a very strong oxidising agent. Why?
Answer:
AgF2 is unstable. So it dissociate into AgF and F. The fluorine liberated is a strong oxidising agent. So AgF2 is strong oxidising agent. The Ag present in AgF2 is in +2 oxidation state. This unstable Ag2+ will be reduced to stable Ag+ during this reaction. As a result, AgF2 acts as a very strong oxidising agent.
Question 42.
Whenever a reaction between an oxidising agent and a reducing agent is carried out, a compound of lower oxidation state is formed if the reducing agent is in excess and a compound of higher oxidation state is formed if the oxidising agent is in excess. Justify the statement giving three illustrations.
Answer:
1) In the reaction between HgCl2 and SnCl2, HgCl2 act as oxidising agent and SnCl2 act as reducing agent. If SnCl2 is excess the product Hg is in its lower oxidation state. But if HgCl2 is excess the product is Hg2Cl2.
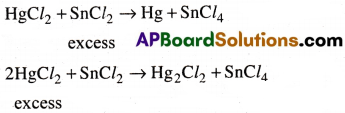
2) In the reaction between phosphorous and chlorine phosphorous is reducing agent and chlorine is oxidising agent. If chlorine is in small amount the product is PCl3 but in the presence of excess chlorine PCl5 is the product.
P4 + 6Cl2 → 4PCl3
P4 + 10Cl2 → 4PCl5
3) When chlorine is passed into excess of liquid sulphur the product is sulphur monochloride S2Cl2 But if excess chlorine is passed until it is saturated, the product is SCl2

Question 43.
How do you count the following observations?
a) Though alkaline potassium permanganate and acidic potassium permanganate both are used as oxidants, yet in the manufacture of benzoic acid from toluene we use alcoholic potassium permanganate as an oxidant. Why? Write balanced redox equation for the reaction.
b) When concentrated sulphuric acid is added to inorganic mixture containing chloride, we get colourless pungent smelling gas HCl, but if the mixture contains bromide then we get vapour of bromine. Why?
Answer:
a) Balanced equation of KMnO4 in acidic medium: MnO–4 +8H+ + 5e– → Mn2+ + 4H2O
Balanced equation of KMnO4 in basic medium: MnO–4 + 2H2O + 3e– → MnO2 + 4OH–
Toluene is oxidised to Benzoic acid in the presence of alc. KMnO4

b) Less volatile acids substitute more volatile acids from the salts. Concentrated sulphuric acid is less volatile and can substitute more volatile HCl and HBr from chlorides and bromides. But HBr is a reducing while HCl cannot act as reducing agent. So sulphuric acid can oxidise the colourless HBr to red vapour of bromine.
2NaCl + H2SO4 → Na2SO4 + 2HCl
2KBr + H2SO4 → K2SO4 + 2HBr
2HBr + H2SO4 → 2H2O + SO2 + Br2
Question 44.
Identify the substance oxidised, reduced, oxidising agent and reducing agent for each of the following reactions:
a) 2AgBr(s) + C6H6O2(aq) → 2Ag(s) + 2HBr(aq) + C6H4O2(aq)
b) HCHO(l) + 2[Ag(NH3)2]+(aq) + 3OH–(aq) → 2Ag(s) + HCOO–(aq) + 4NH3(aq) + 2H2O(l)
c) HCHO(l) + 2Cu2+(aq) + 5OH–(aq) → Cu2O(s) + HCOO–(aq) + 3H2O(l)
d) N2H4(l)+ 2H2O2(l) → N2(g) + 4H2O(1)
e) Pb(s) + PbP2(s) + 2H2SO4(aq) → 2PbSO4(s) + 2H2O(l)
Answer:

Oxidised Substance → C6H6O2
Reduced Substance → AgBr
Oxidising agent → AgBr
Reducing agent → C6H6O2

Oxidised Substance → HCHO
Reduced Substance → [Ag(NH3)2]+
Oxidising agent → [Ag(NH3)2]+
Reducing agent → HCHO
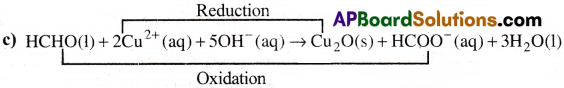
Oxidised Substance → HCHO
Reduced Substance → Cu+2
Oxidising agent → Cu+2
Reducing agent → HCHO
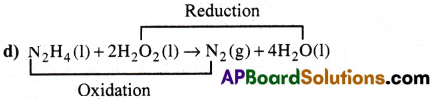
Oxidised Substance → N2H4
Reduced Substance → H2O2
Oxidising agent → H2O2
Reducing agent → N2H4
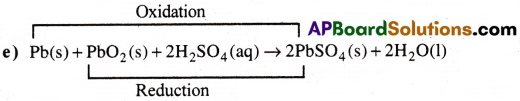
Oxidised Substance → Pb
Reduced Substance → PbO2
Oxidising agent → PbO2
Reducing agent → Pb
Question 45.
Consider the reactions
2S2O2-3(aq) + I2(s) → S4O2-6(aq) + 2I–(aq)
S2O2-3(aq) + 2Br2(l) + 5H2O(l) → 2SO2-4(aq) + 4Br–(aq) + 10H+(aq)
Why does the same reductant, thiosulphate react differently with iodine and bromine?
Answer:
Iodine is a weak oxidising agent while bromine is stronger oxidising agent. So the oxidation of
S2O2-3 with iodine will take place until the oxidation state of sulphur +2 in S2O2-3 changes to 2.5 in S4O2-6 only- But bromine being stronger oxidising agent can oxidise the sulphur iron S2O2-8 to its highest oxidation state +6 in SO2-4.
![]()
Question 46.
Justify giving reactions that among halogens, fluorine is the best oxidant and among hydrohalic compounds, hydroiodic acid is the best reductant.
Answer:
Among halogens oxidation power decreases from fluorine to iodine due to decrease in electro negatives and electron gain enthalpies. This can be explained as follows.
Fluorine can displace Cl2, Br2 and I2 from the corresponding halides.
2KCl + F2 → 2KF + Cl2
2KBr + F2 → 2KF + Br2
2KI + F2 → 2KF + I2
Chlorine can displace Br2 and I2 from bromides and iodides respectively but cannot displace fluorine from fluorides.
2KBr + Cl2 → 2KCl + Br2
2KI + Cl2 → 2KCl + I2
Bromine can displace I2 from iodide but cannot displace F2 from fluorides or Cl2 from chlorides.
2KI + Br2 → 2KBr + I2
Iodine cannot displace any other halogen from their halides.
In the hydrogen halides the reduction power increases from HF to HI . This is because of the decrease in thermal stability of hydrogen halides with increases in bond length. Further the tendency to hold the electron decreases from HF to HI. So HF cannot be oxidised but HI can be easily oxidised. Hence HI is the best reductant.
Question 47.
Why does the following reaction occur?
XeO4-6(aq) + 2F–(aq) + 6H+(aq) → XeO3(g) + F2(g) + 3H2O(l)
What conclusion about the compound Na4XeO6 (of which is a part) can be drawn from the reaction.
Answer:
The perxenate ion XeO4-6 ion is very strong oxidising agent than fluorine. So it can oxidise F– ion to fluorine in acid medium. Hence the reaction occurs.
XeO4-6(aq) + 2F–(aq) + 6H+(aq) —> XeO3(g) + F2(g) + 3H2O(l)
Hence, we conclude that XeO4-6 (or) Na4XeO6 is a stronger oxidising agent than F2.
Question 48.
Consider the reactions:
a) H3PO2(aq) + 4AgNO3(aq) + 2H2O(l) → H3PO4(aq) + 4Ag(s) + 4HNO3(aq)
b) H3PO2(aq) + 2CuSO4(aq) + 2H2O(l) → H3PO4(aq) + 2Cu(s) + H2SO4(aq)
c) C6H5CHO(l) + 2[Ag(NH3)2]+(aq) + 3OH–(aq) → C6H5COO–(aq) + 2Ag(s) + 4NH3(aq) + 2H2O(l)
d) C6H5CHO(l) + 2Cu2+(aq) + 5OH–(aq) → no change is observed.
Answer:
From (a) and (b) it is clear that Ag+ and Cu2+ both act as oxidising agents.
In alkaline medium Ag+ is oxidising benzaldehyde to benzoate but Cu2+ has no action. This indicates that in alkaline medium Ag+ is stronger oxidising agent than Cu2+.
Question 49.
Balance the following redox reactions by ion-electron method.
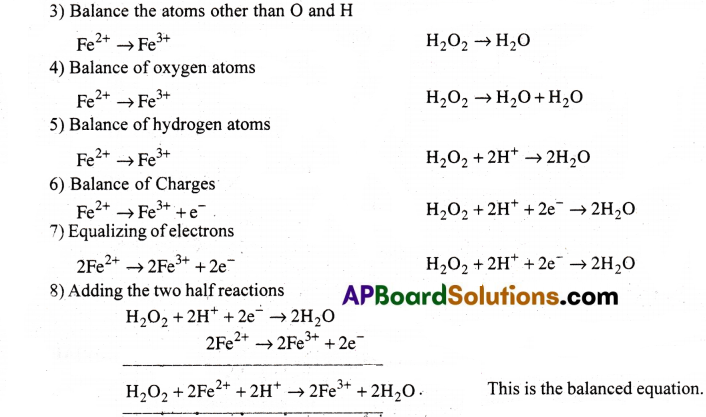
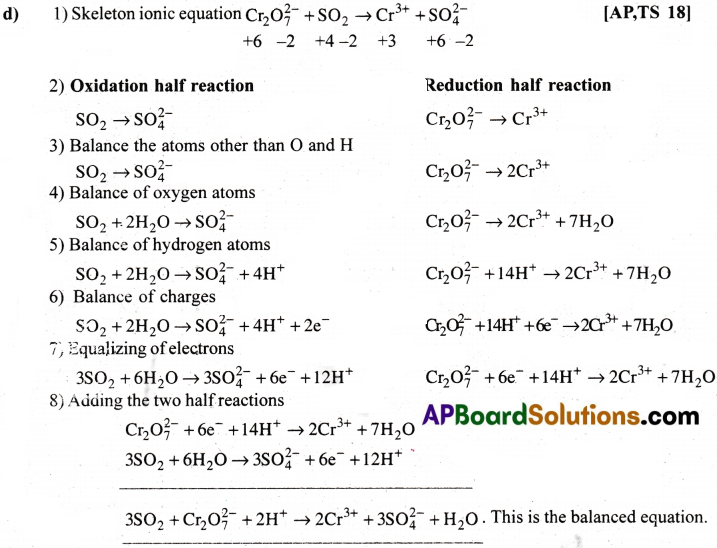
a) MnO–4(aq) + I–(aq) → MnO2(s) + I2(s)(in basic medium) [AP 19]
b) MnO–4(aq) + SO2(g) → Mn2+(aq) + HSO4(aq)(in acidic solution) [AP 15][TS 22]
c) H2O2(aq) + Fe2+(aq) → Fe3+(aq) + H2O(l) (ion acidic solution)
d) Cr2O2-7 + SO2(g) → Cr3+(aq) + SO2-4(aq)(ion acidic solution)
Answer:

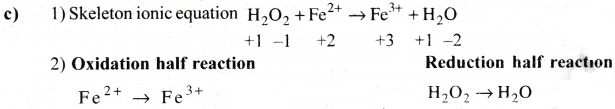
Question 50.
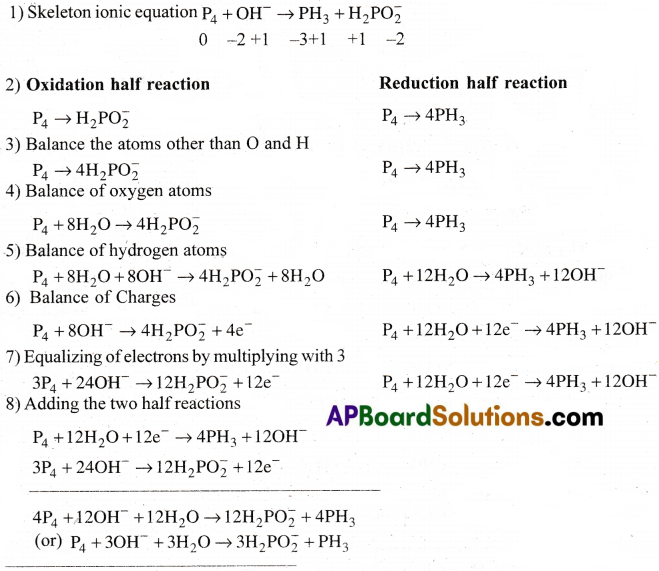
Balance the following equations in basic medium by ion-electron method and oxidation number methods and identify the oxidising agent and the reducing agent.
a) P4(s) + OH–(aq) → PH3(g) +H2PO–2(aq)
b) N2H4(l) + ClO–3(aq) → NO(g) + Cl–(g)
c) Cl2O7(g) + H2O2(aq) → ClO–2(aq) + 02(g) + H+
Answer:
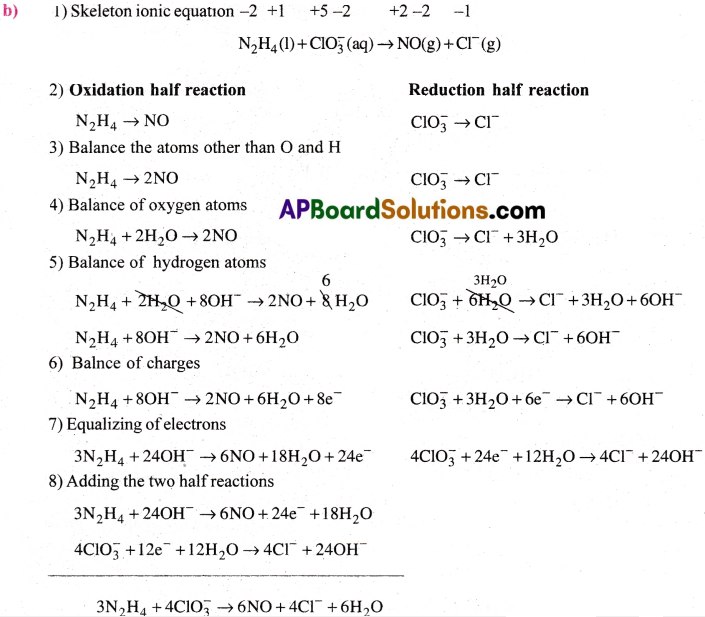
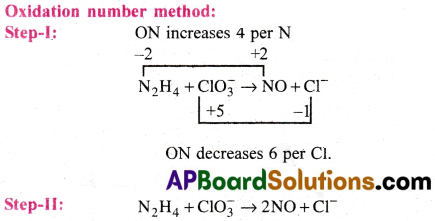
Step-III: Equalise the increase and decrease in ON by multiplying N2H4 with 3 and ClO–3 with 2.
3N2H4 + 4ClO–3 → 6NO + 4Cl–
Step-IV: Balance the atoms except H and O. Here they are balanced.
Step-V: Balance O atoms by adding OH– ions and H atoms by adding H2O on the sides, deficient of O and H atoms respectively.
3N2H4 + 4ClO–3 → 6NO + 4Cl– + 6H2O
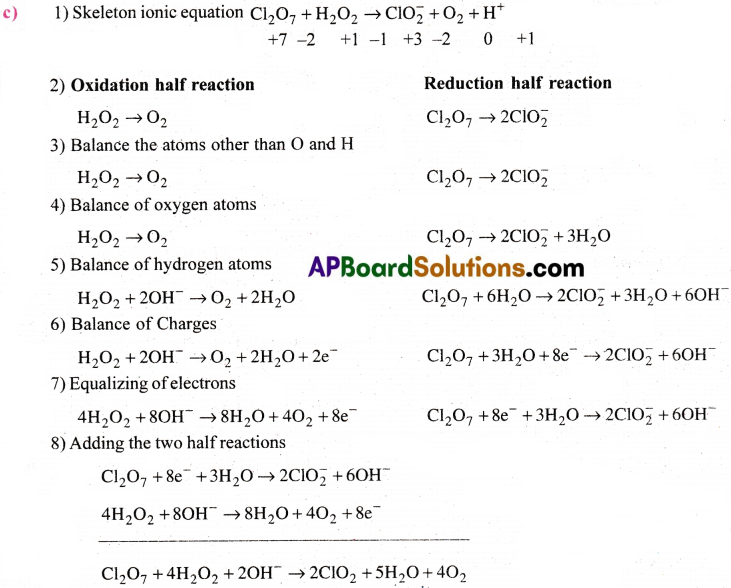
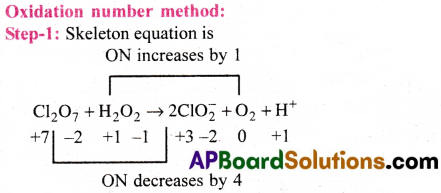
Step-II: Equalise the increases /decrease in ON by multiply H2O2 with 4 since in each chlorine of Cl2O7 decrease in ON is 4. For 2 Cl atoms it is 8. In H2O2 increase in ON for each 0 is 1 and for two 0 atoms it is 2.
Cl2O7 + 4H2O2 → 2ClO–2 + 4H2O + 2O2
Step-III: Balance the O atoms by adding OH– and H atoms by adding H2O to the sides deficient of O and H atoms respectively.
Cl2O7 + 4H2O2 + 2OH– → 2ClO–2 + 5H2O + 4O2
Question 51.
What sorts of informations can you draw from the following reaction?
(CN)2(g) + 2OH–(aq) → CN–(aq) + CNO–(aq) + H2O(l)
Answer:
The oxidation numbers of carbon in (CN)2, CN– and CNO– are +3, +2 and +4 respectively.
The oxidation number of carbon in the various species is.
![]()
It can be easily observed that the same compound is being reduced and oxidised simultaneously. So it is disproportionation reaction.
Decomposition of cyanogen in alkali medium is disproportion atom reaction.
Question 52.
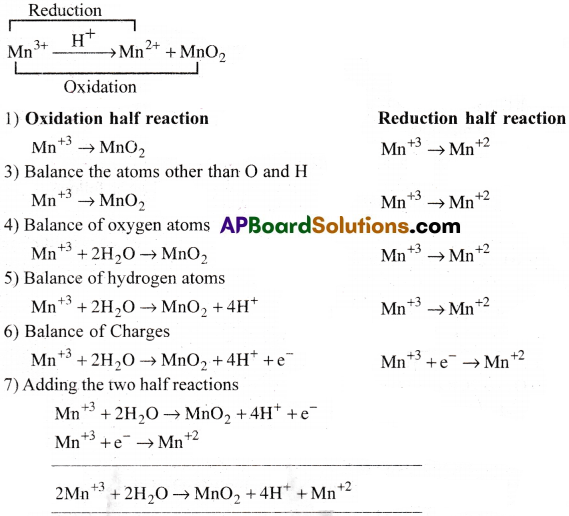
The Mn3+ ion is unstable solution and undergoes disproportionation to give Mn2+, MnO2 and H+ ion. Write balanced ionic equation for the reaction.
Answer:
Question 53.
Consider the elements Cs, Ne, I and F.
a) Identify the element that exhibits only negative oxidation state.
b) Identify the element that exhibits only positive oxidation state.
c) Identify the element that exhibit both positive and negative oxidation state.
d) Identify the element which neither the negative nor does the positive oxidation state.
Answer:
a) ’F’ exhibit only negative oxidation states because it is the most electronegative atom,
b) ‘Cs’ exhibit only positive oxidation state because it is the most electropositive element.
c) T can exhibit both positive and negative oxidation states:
Ex: In ICl3 the oxidation state of I is +3 and in Nal oxidation state of I is -1.
d) Ne being inert gas do not participate in reactions. So it while not exhibit neither the negative nor the positive oxidation states.
Question 54.
Chlorine is used to purify drinking water. Excess of chlorine is harmful. The excess of chlorine is removed by treating with sulphur dioxide. Present a balanced equation for this redox change taking place in water.
Answer:
SO2 + Cl2 + 2H2O → H2SO4 + 2HCl
Question 55.
Refer to the periodic table given in your book and now answer the following questions.
a) Select the possible non metals that can show disproportionation reaction.
b) Select the metals that can show disproportionation.
Answer:
a) Phosphorous, Sulphur, Chlorine, bromine, iodine,
b) Copper, silver, gold.
Question 56.
In Ostwald’s process for the manufacture of nitric acid the first step involves the oxidation of ammonia gas by oxygen gas to give nitric oxide gas and steam. What is the maximum weight of nitric oxide that can be obtained starting only with 10.00 g of ammonia and 20.00 g of oxygen.
Answer:
The oxidation of ammonia to NO in Ostwalds process can takes place as follows.
4NH3 + 5O2 → 4NO + 6H2O
68 gm of ammonia react with 160 gm of Oxygen. In this reaction oxygen is limiting reagent. Since to react with 10 g of ammonia the required amount of oxygen is
\(\frac{10\times160}{68}\) = 23.53gm of oxygen is required
But there is only 20.00 g of oxygen.
160 gm of O2 can react with 68 gm of NH3
∴ 20 gm of O2 can react with \(\frac{20\times68}{160}\) = 8.5gm NH3
For 68 gm of NH3 the wt. of NO formed is 120.
Fro 8.5 gm of NH3 the wt of NO formed is 15 gm.
Question 57.
Arrange the following metals in the order in which they displace each other from the solution of their salts. Al, Cu, Fe Mg and Zn.
Answer:
A metal of stronger reducing power displaces another metal of weaker reducing power from its solution of salt.
The order of the increasing reducing power of the given metals is Cu<Fe<Zn<Al<Mg
Hence we can say that Mg can displace Al from it’s salt solution, but all cannot displace Mg. Thus, the order in which the given metals displace each other from the solution of their salts is given below.
Mg>Al> Zn>Fe>Cu.
Long Answer Questions
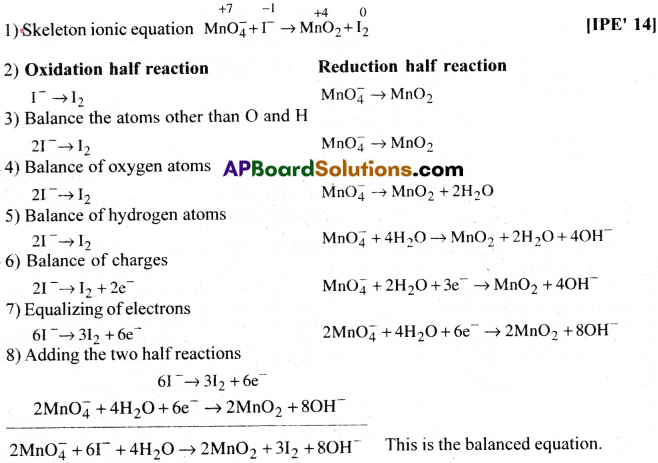
Question 1.
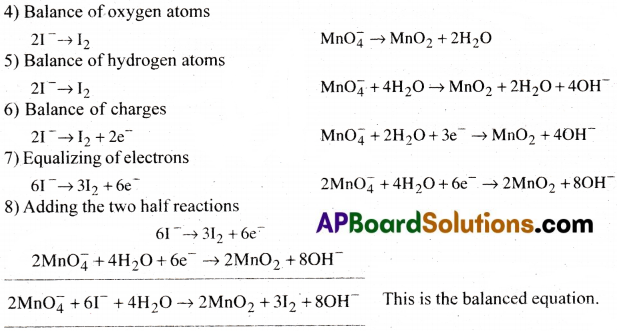
Write the balanced ionic equation which represents the oxidation of iodide (I–) ion by permanganate ion in basic medium to give iodine (l2) and manganese dioxide (MnO2).
Answer:
Question 2.
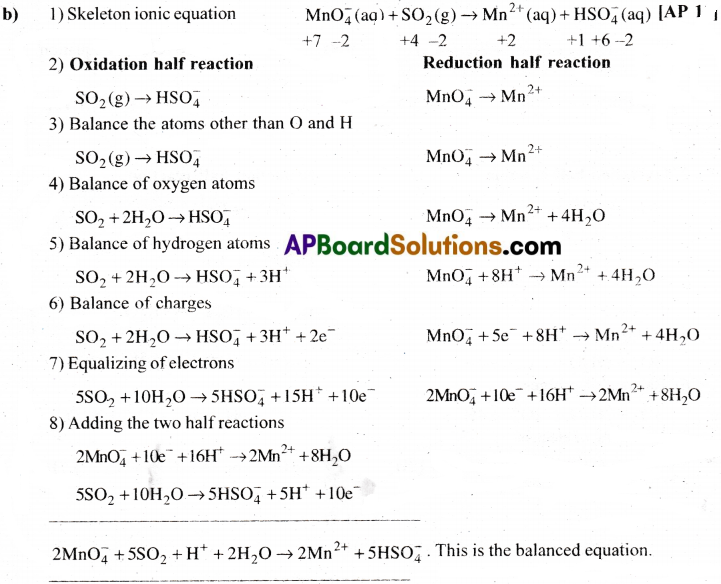
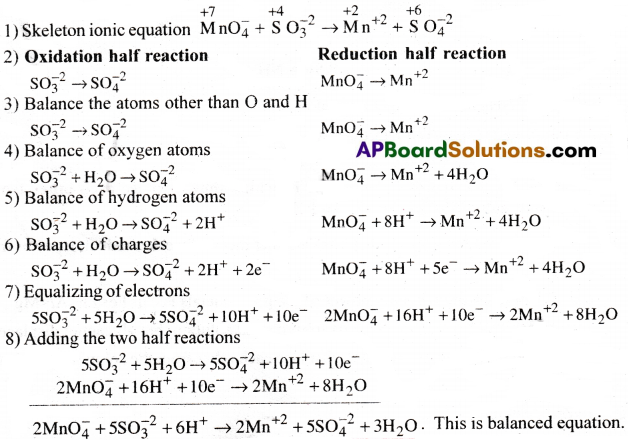
Balance the reaction MnO–4 + SO-23 → Mn+2 + SO-24 in acid medium (Or)
Write the balanced equation for the oxidation of sulphite ions to sulphate ions in acid medium by permanganate ion.
Answer:
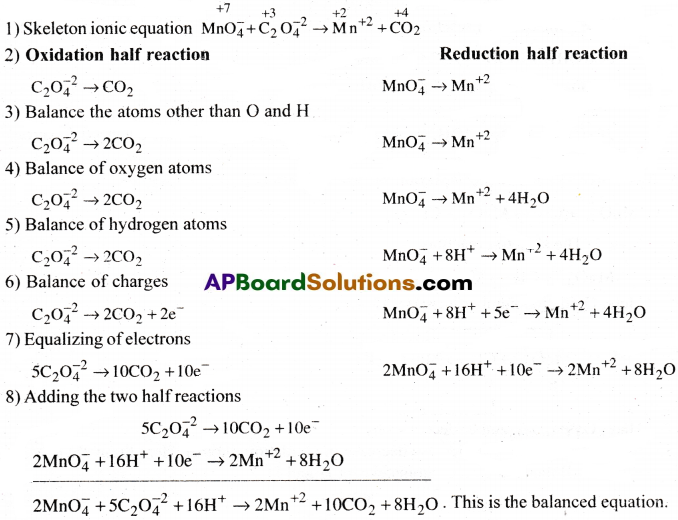
Question 3.
Oxalic acid is oxidized by permanganate ion in acid medium of Mn+2 balance the reaction by ion-electron method.
Answer:
![]()
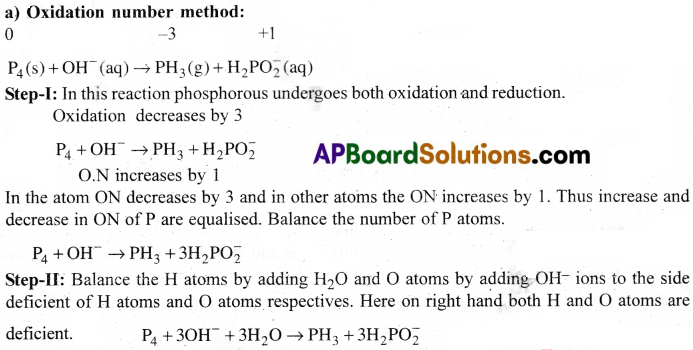
Question 4.
Phosphorus when heated with NaOH solution gives phosphine (PH3) and H2PO2. Give the balanced equation.
Answer:




Step-III: Equalise the increase and decrease in ON by multiplying N2H4 with 3 and ClO–3 with 2.
3N2H4 + 4ClO–3 → 6NO + 4Cl–
Step-IV: Balance the atoms except H and O. Here they are balanced.
Step-V: Balance O atoms by adding OH– ions and H atoms by adding H2O on the sides, deficient of O and H atoms respectively.
3N2H4 + 4ClO–3 → 6NO + 4Cl– + 6H2O


Step-II: Equalise the increases /decrease in ON by multiply H2O2 with 4 since in each chlorine of Cl2O7 decrease in ON is 4. For 2 Cl atoms it is 8. In H2O2 increase in ON for each 0 is 1 and for two 0 atoms it is 2.
Cl2O7 + 4H2O2 → 2ClO–2 + 4H2O + 2O2
Step-III: Balance the O atoms by adding OH– and H atoms by adding H2O to the sides deficient of O and H atoms respectively.
Cl2O7 + 4H2O2 + 2OH– → 2ClO–2 + 5H2O + 4O2
Question 5.
Balance the following equation
![]()
Answer:
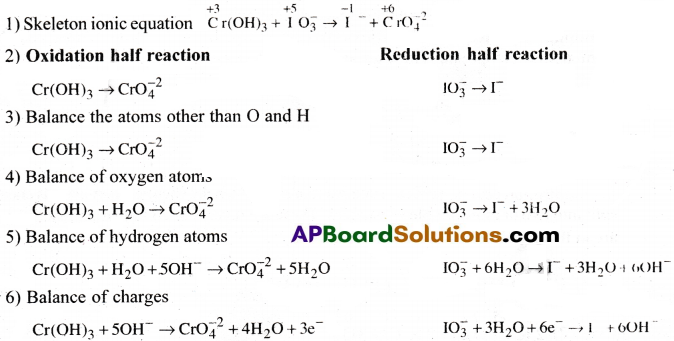
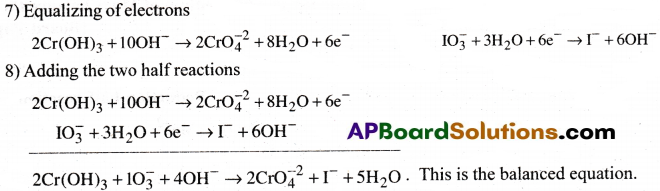
Question 6.
Balance the following equation by the oxidation number method.
MnO2-4 + Cl2 → MnO–4 + Cl–
Answer:
Step-1: The skeleton reaction
MnO2-4 + Cl2 → MnO–4 + Cl–
Here, Oxidation number increases by 1
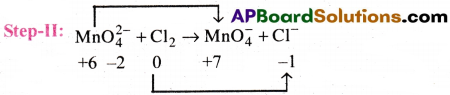
Here, Oxidation number decreases by 1
Step-III : Equalise the increase/decrease in Oxidation number. Here these are equal.
Step-IV : Balance the other atoms except H and O
2MnO2-4 + Cl2 → 2MnO–4 + 2Cl–
Step-V: Balance H atoms and O atoms. Here they are balanced.
The balanced equation is
2MnO2-4 +Cl2 → 2MnO–4 + 2Cl–
Question 7.
Explain the different types of redox reactions.
Answer:
A chemical reaction in which both oxidation and reduction reactions are involved is called an a redox reaction (or) oxidation-reduction reaction or simply
1) Combination reactions :
The redox reactions, in which two or more elements combine to form a new compound. These are in the form A + B → AB

2) Decomposition reactions :
The redox reactions, in which the chemical compounds chemically split into two or more simple substances. These are reverse of combination reactions. These are in the form AB → A + B.

3) Displacement reactions :
The redox reactions, in which an ion (atom) in a compound is replaced by an ion (atom) of another element. These are in the form A + BC → AC + B
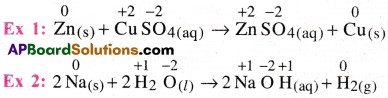
4) Disproportionation reactions :
The redox reactions, in which an element in one oxidation state is simultaneously oxidised and reduced.
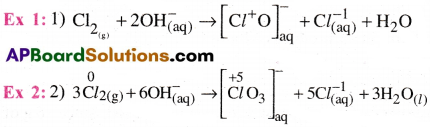
5) Comproportionation reaction :
In this reactions, two species with the same element in two different oxidation states form a single product, in which the element is in an intermediate oxidation state.These are the reverse of disproportonaion reactions.
Ex 1: Cu+2(aq) + Cu (s) → 2Cu+(aq)
Ex 2: Ag2+(aq) + Ag(s) → 2Ag+(aq)
![]()
Question 8.
State the law of definite proportions. Suggest one problem to understand the law by working out that problem.
Answer:
Law of definite proportions:
“A given chemical substance always contains the same elements combined in a fixed proportion by weight.”
Explanation:
SO2 can be obtained by the following two methods.
i) When mercuric sulphide is heated in air, it gives mercury and sulphur dioxide.
HgS + O2 → Hg + SO2
ii) When lead sulphide is heated strongly in air, it gives lead oxide and sulphur dioxide.
2PbS + 3O2 → 2PbO + 2SO2
Samples of SO2 obtained by the aobve two methods were analysed. In each of them, 100 g of SO2 was found to contain same percent by weight of sulpur and oxygen.
The above observations prove that the weight composition of sulphur dioxide is always constant.
Question 9.
How are the end points of titrations detected in the following reactions?
a) MnO2-4 oxidises Fe2+
b) Cr2O2-7 oxidises Fe2+
c) Cu+2 oxidises I–
Answer:
a) In the oxidation of Fe2+ with MnO–4 the permanganate itself act as self indicator. MnO–4 has purple colour. The visible end point in this case is achieved after the last amount reductant (Fe2+) is oxidised and the first stable tinge of pink colour appears.
b) In the oxidation of Fe2+ with Cr2O2-7 an indicator such as diphenyl amine is used. Just after the equivalence point the excess Cr2O2-7 oxidises the diphenyl amine to intence blue colour by which the end point can be detected.
c) In the oxidation of I– with Cu2+ the iodine formed will give intense blue colour with starch. This colour will be discharged with excess of hypo added after the equivalence point.
Question 10.
Calculate the amount of Carbondioxide that could be produced when
i) 1 mole of carbon is burnt inair
ii) 1 mole of carbon is burnt in 16g of dioxvgen.
iii) 2 moles of carbon are burnt in 16 g of dioxvgen. [AP 19]
Answer:

For burning 12 g (1 mole) of carbon 32 gm of dioxygen is required. Since 16 g of dioxygen is present only 6 gm (half mole) of carbon bum producing half mole of CO2.
Thus 22 g of CO2 is formed.
iii) Here also 22 g of CO2 is formed since there is only 16 g of oxygen.
Question 11.
Dinitrogen and dihydrogcn react with each other to produce ammonia according to the following chemical equation.
N2(g) + H2(g) → 2NH3(g)
i) Calculate the mass of ammonia produced if 2.00 × 10³ g dinitrogen reacts with 1.00 × 10³ g of dihydrogen.
ii) Will any of the two reactants remain unreacted.
iii) If yes, w hich one and what would be its mass.
Answer:
i) The balanced equation for the reaction between dihydrogen and dinitrogen is
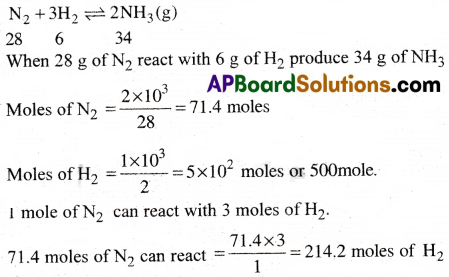
Here 1 mole of N2 can produce 34 g of NH3.
71.4 mole of N2 can produce
71.4 × 34 = 2427.6 gm.
iii) Here No. of moles of H2 are more than required
The no.of moles of H2 unreacted = 500 – 214.2 = 285.8
The amount of hydrogen left = 285.8 × 2 = 571.6 gm.
Question 12.
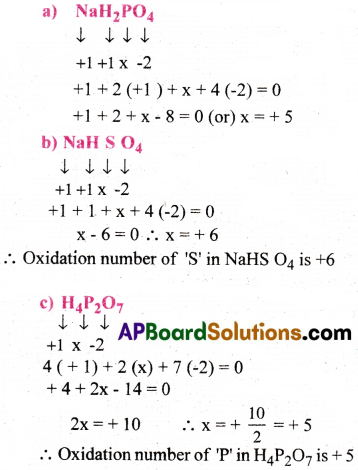
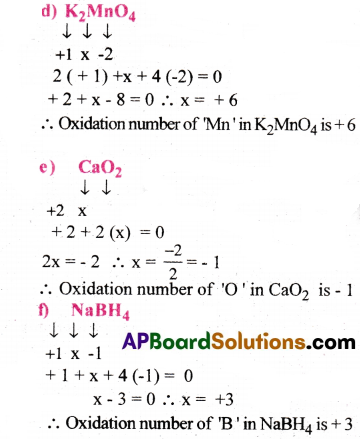
Assign Oxidation number to the underlined elements in each of the following species:
a)NaH2PO4
b) NaHSO4
c) H4P2O7
d) K2MnO4
e) CaO2
f) NaBH4
g) H2S2O7
h) KAI (SO4)2.12H2O
Answer:

Question 13.
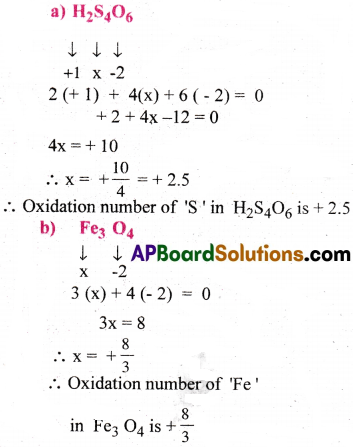
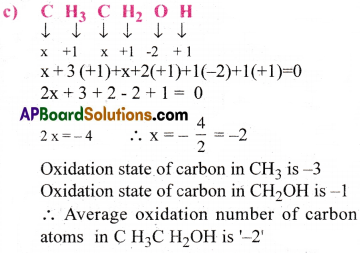
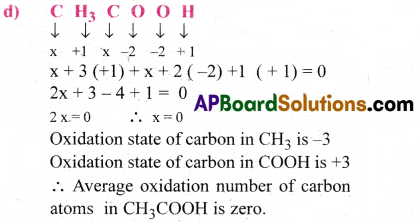
What are the oxidation numbers of the underlined elements in each of the following and how do you rationalize your results?
a) H2S4O6
b) Fe3O4
c) CH3CH2OH
d) CH3COOH
Answer:
Textual Solved Problems
Question 1.
Calculate molecular mass of glucose (C6H|2Og) molecule.
Solution:
Molecular mass of glucose (C6H12O6) = 6(12.011) +12 (1.008) + 6 (16.00)
= (72.066) + (12.096) + (96.00) = 180.162 g
![]()
Question 2.
A compound contains 4.07 % hydrogen, 24.27% carbon and 71.65% chlorine. Its molar mass is 98.96 g. What are its empirical and molecular formulas? [TS 17,19]
Solution:
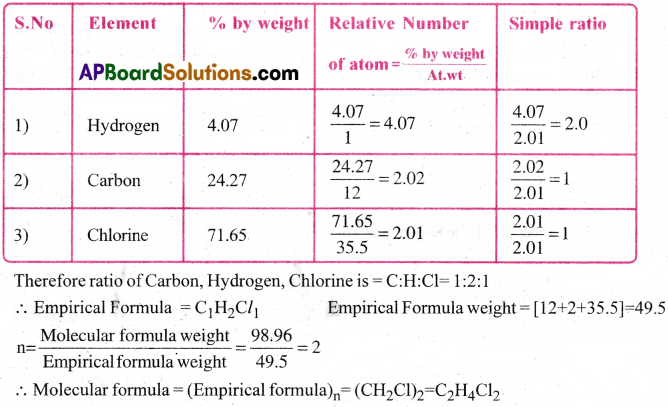
Question 3.
Calculate the amount of water (g) produced by the combustion of 16 g of methane.
Solution :
The balanced equation for combustion of methane is
CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
(i) 16 g of CH4 corresponds to one mole.
(ii) From the above equation, 1 mol of CH4 (g) gives 2 mol of H2O(g).
2 mol of water (H2O) = 2 × (2 + 16) = 2 × 18 = 36 g
Question 4.
How many moles of methane are required to produce 22 g CO2 (g) after combustion?
Solution:
According to the chemical equation. CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
44 grh of CO2 is obtained – 1 mole of CH4 is
22 gm of CO2 is obtained – ? required = \(\frac{22\times1}{44}\) = 0.5 moles of CH4 is required.
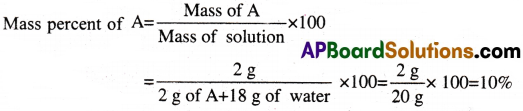
Question 5.
A solution is prepared by adding 2 g of a substance A to 18 g water. Calculate the mass percent of the solute. [TS 19][AP 15]
Solution:
Question 6.
Calculate the molarity of NaOH in the solution prepared by dissolving 4 g in enough water to form 250 ml of the solution. [AP 18] [TS 16]
Solution:
Wt. of NaOH = 4gm, V = 250ml, GMW = 40, M = ?
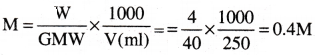
Question 7.
The density of 3 M solution of NaCl is 1.25 g mL-1. Calculate molality of the solution.
Solution:
M = 3 mol L-1. Mass ofNaCl in 1 L solution = 3 × 58.5 = 175.5 g
Mass of 1 L solution = 1000 × 1.25 = 1250 g (since density = 1.25 g mL-1)
Mass of water in solution = 1250 – 175.5 = 1074.5 g = 1.0745 kg.
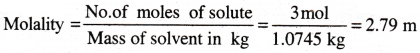
![]()
Question 8.
Calculate the normality of oxalic acid solutions containing 6.3 g of H2C2O4. 2H2O in 500 ml of solutions. [AP 20][TS 15]
Solution:
Weight of solute = 6.3g

Question 9.
Calculate the mass of Na2CO3 required to prepare 250 ml of 0.5 N solution.
Solution:
Normality of required solution = 0.5 N;
Volume of required solution = 250 ml

Multiple Choice Questions
Question 1.
Which of the following is not an example of redox reaction?
1) CuO + H2 → Cu + H2O
2) Fe2O3 + 3CO → 2Fe + 3CO2
3) 2K + F2 → 2KF
4) BaCl2 + H2SO4 → BaSO4 + 2HCl
Answer:
4) BaCl2 + H2SO4 → BaSO4 + 2HCl
Quetion 2.
In which of the following compounds, an element exhibits two different oxidation states.
1) NH2OH
2) NH4NO3
3) N2H4
4) N3H
Answer:
2) NH4NO3
Question 3.
Which of the following elements does not show disproportionation tendency?
1) Cl
2) Br
3) F
4) I
Answer:
3) F
Question 4.
Which of the following outer electronic configurations the element will exhibit largest oxidation number?
1) 3d¹4s²
2) 3d³4s²
3) 3d54s¹
4) 3d54s²
Answer:
4) 3d54s²
Question 5.
Which of the following reactions is the metal displacement reaction? Choose the right option.
Answer:
3
Question 6.
Which of the following arrangements represent increasing oxidation number of the central atom?
1) CrO–2, ClO–3, CrO2-4, MnO–4
2) ClO–3, CrO2-4, MnO–4, CrO2-4
3) CrO–2, ClO–3, MnO–4, CrO2-4
4) CrO2-2, MnO–4, CrO–2, ClO–3
Answer:
1) CrO–2, ClO–3, CrO2-4, MnO–4
![]()
Question 7.
What is the change in oxidation number of carbon in the following reaction?
CH4(g) + 4Cl2(g) → CCl4(l) + 4HCl(g)
1) +4 to +4
2) 0 to +4
3) -4 to +4
4) 0 to -4
Answer:
3) -4 to +4
Question 8.
The correct order of N-compounds in its decreasing order of oxidation states is
1) HNO3, NO, N2, NH4Cl
2) HNO3, NO, NH4Cl,N2
3) HNO3, NH4Cl,NO, N2
4) NH4Cl, N2,NO, HNO3
Answer:
1) HNO3, NO, N2, NH4Cl
Question 9.
For the redox reaction,
MnO–4 + C2O2-4 + H+ → Mn2+ + CO2 + H2O
The correct coefficients of the reactants for the balanced equation are MnO–4, C2O2-4 , H+

Answer:
2
Question 10.
Hot concentrated sulphuric acid is a moderately strong oxidizing agent. Which of the following reactions doest not show oxidizing behaviour?
1) Cu +2H2SO4 → CuSO4 + SO2 + 2H2O
2) S + 2H2SO4 → 3SO2 + 2H2O
3) C + 2H2SO4 → CO2 + 2SO2 + 2H2O
4) CaF2 + 2H2SO4 → CaSO4 + 2HF
Answer:
4) CaF2 + 2H2SO4 → CaSO4 + 2HF
Question 11.
Which of the following reactions are disproportionation reactions?

Select the correct option from the following:
1) i and iv only
2) i and ii only
3) i, ii and iii
4) i, iii and iv
Answer:
2) i and ii only
Question 12.
The number of atoms present in one mole of an element is equal to Avogadro number. Which of the following element contains the greatest number of atoms?
1) 4g He
2) 46g Na
3) 0.40g Ca
4) 12g He
Answer:
4) 12g He
![]()
Quetion 13.
One mole of any substance contains 6.022 × 1023 atoms/molecules. Number of molecules of H2SO4 present in 100 mL of 0.02INI H2SO4 solution is ____
1) 12.044 × 1020 molecules
2) 6.022 × 1023 molecules
3) 1 × 1023molecules
4) 12.044 × 1023 molecules
Answer:
1) 12.044 × 1020 molecules
Question 14.
What is the mass percent of carbon in carbon dioxide?
1) 0.034%
2) 27.27%
3) 3.4%
4) 28.7%
Answer:
2) 27.27%
Question 15.
The empirical formula and molecular mass of a compound are CH2O and 180 g respectively. What will be the molecular formula of the compound?
1) C9H18O9
2) CH2O
3) C6H12O6
4) C2H4O2
Answer:
3) C6H12O6
Question 16.
What will be the molarity of a solution, which contains 5.85 g of NaCl(s) per 500 mL?
1) 4 mol L-1
2) 20 mol L-1
3) 0.2 mol L-1
4) 2 mol L-1
Answer:
3) 0.2 mol L-1
Question 17.
If 500 mL of a 5M solution is diluted to 1500 mL, what will be the molarity of the solution obtained?
1) 1.5 M
2) 1.66 M
3) 0.017 M
4) 1.59 M
Answer:
2) 1.66 M
Question 18.
If the concentration of glucose (C6H12O6) in blood is 0.9 g L-1 what will be the molarity of glucose in blood?
1) 5 M
2) 50 M
3) 0.005 M
4) 0.5 M
Answer:
3) 0.005 M
Question 19.
What will be the molality of the solution containing 18.25 g of HCl gas in 500 g of water?
1) 0.1 m
2) 1 M
3) 0.5 m
4) 1 m
Answer:
4) 1 m
![]()
Question 20.
If the density of a solution is 3.12 g mL-1, the mass of 1.5 mL solution in significant figures is ____
1) 4.7g
2) 4680 × 10-3g
3) 4.680g
4) 46.80g
Answer:
1) 4.7g