Students can use TS 10th Class English Model Papers and TS 10th Class English Question Paper April 2023 as a tool for exam preparation.
TS 10th Class English Question Paper April 2023
Time: 3 Hours
Maximum Marks: 80
General Instructions:
- Read the question paper carefully.
- Answer the questions under Part – A in the answer booklet provided.
- Write the answer to the questions under Part – B on the question paper itself.
- Avoid overwriting.
Part – A (60 Marks)
Question No. (1 – 4) : Read the following passage.
‘When I was 13, I read a newspaper article about a disabled man who had managed to achieve great things and helped others, ’said Nick.
‘I realised why God had made us like this – to give hope to others. It was so inspirational to me that I decided to use my life to encourage other people and give them the courage that article had given me.’
‘I decided to be thankful for what I do have, not get angry about what I don’t’.
‘I looked at myself in the mirror and said: ‘You know what the world is right that I have no arms or legs, but they’ll never take away the beauty of my eyes. ‘I wanted to concentrate on something good that I had.’
“The challenges in our lives are there to strengthen our convictions. They are not there to run us over,” said Nick. In 1990 Nick won the Australian Young Citizen of the Year award for his bravery and perseverance. (Attitude is Altitude)
Answer each of the following questions in three to four sentences. 4 × 3 = 12 M
Question 1.
What change did the newspaper article bring in Nick?
Answer:
After reading the newspaper article there was a great change in Nick’s attitude. A pessimist changed into an optimist. Nick who hated God for making him a torso realised that God made him so to give hope to others. The article was so inspirational to him that he decided to use his life to encourage other people and give them the courage that the article had given him.
Question 2.
‘I decided to be thankful for what I do have, not get angry about what I don’t’, -is it applicable to Nick ? Explain.
Answer:
Yes, it is applicable to Nick. The newspaper article changed his attitude. Before reading the article he was a pessimist. He even tried to end his life by committing suicide. He hated God for making him a torso. After reading the article he became an optimist. He realised the purpose of his life. He realised that God made him a torso to give hope to others. He decided to use his life to encourage others. Hence he decided to be thankful for what he had, not get angry about what he didn’t have.
Question 3.
According to Nick, how can we overcome the challenges in our life ?
Answer:
Nick feels that the challenges in our lives are there to strengthen our convictions. They are not there to run us over. According to Nick, we can overcome challenges in our life with a positive attitude, optimistic nature, self-confidence, bravery and perseverance.
Question 4.
If you were Nick, how would you face the challenges in your life ? Explain.
Answer:
The life of Nick Vujicic teaches Us to have confidence on ourselves. It is a motivation for physically challenged people. There is none who is less than the others. Despite various difficulties everyone can achieve goals.
If I were Nick, I would face the challenges in my life efficiently. With positive traits such as never-give up attitude, optimism, bravery and perseverance. I would overcome the challenges in my life.
![]()
Question No. (5 – 8) : Read the following passage.
There is little else one could expect when all time greats like Marcus Bartley (cinematography), Ghantasala (music), M.L. Vasantha Kumari, Leela, Suseela and Madfravapeddi (playback), Gokhale (art), Pasumarthy (choreography) and Pitambaram (make-up) got together to weave magic around an episode from Mahabharatha, Sasirekha parinayam.
However, the greatness of Maya Bazaar, about which much is said and written, is hot just because of these facets alone.
It is a tribute to Telugu culture, language and customs of the land. The film was watched repeatedly soon after its release because people identified every character of the film with someone they knew in their immediate vicinity and the audience still do the same now.
The dialogues written by Pingali Nagendra Rao (as well the lyrics) were the same that the people were hearing or using in their conversations every day – if not, those became a part of Telugu life thereafter. Sasirekha’s, nay Ghatothkacha’s Manadi Sodara Prema ……………. became immortalized as much as Suryakantam’s antha alamalame kada which has become a way of life in greeting people.
As for songs, Aha naa pelli anta still reverberates in marriages and Vivaaha bhojanambu is yet another must.
Answer each of the following questions in three to four sentences. 4 × 3 = 12 M
Question 5.
flow do you feel when you identify yourself with one of the characters in a movie ?
Answer:
In any movie some characters possess positive qualities while some have negative qualities. Characters with positive qualities cause a good impression and have a lasting impact on the audience. They show their love, affection, sympathy, compassion, etc. towards characters with positive qualities. So I would like to identify myself with a character possessing positive qualities so that I would feel happy, satisfied and proud.
Question 6.
How did the dialogues in the movie ‘Maya Bazaar’ influence the audience ?
Answer:
The dialogues in the movie ‘Maya Bazaar’ have strong influence on the Telugu audience. Many of the dialogues are still being heard or used in their conversations every day. They became a part of Telugu people’s life.
Question 7.
How did the ‘Maya Bazaar’ reflect our lifestyle ?
Answer:
The movie ‘Maya Bazaar’ reflected our lifestyle greatly. It is a tribute to Telugu culture, language and customs of the land. Telugu people identified every character of the film with someone they knew in their immediate vicinity. The dialogues and songs in the movie became a part of Telugu life. Many of the dialogues became immortalized. As for songs, ‘Aha naa pelli anta’ and ‘Vivaha Bhojanambu’ still reverberate in marriage.
Question 8.
Pick Three TRUE statements according to the passage.
A) Maya Bazaar is based on the complete story of the Maha Bharatha.
B) Pingali Nagendra Rao wrote dialogues and songs for the movie.
C) The language used in the language of every day.
D) The characters in the movie represent the royal class of the society.
E) Every artist contributed to the perfection of Maya Bazaar.
Answer:
True statements: B, C, E
![]()
Question No. (9 – 12): Study the table carefully and answer the questions given. 4 × 2 = 8 M
Musical Instruments
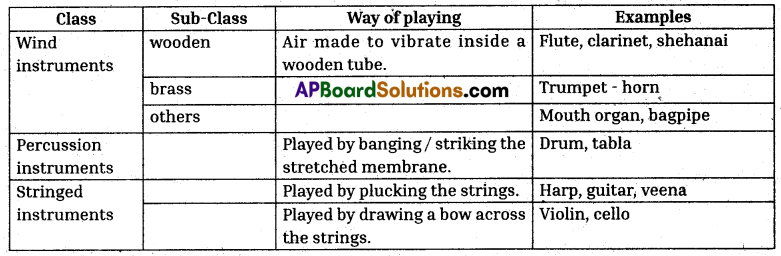
Question 9.
What is the table mainly about ? How many categories and sub-categories are mentioned ?
Answer:
The table is mainly about Musical Instruments. Three categories and three sub-categories are mentioned in the table.
Question 10.
How is a clarinet different from a mouth organ and trumpet ?
Answer:
All the three instruments belong to the same class, i.e., wind instruments. The clarinet is a member of the woodwind family whereas the trumpet is the member of the brass family. Mouth organ belongs to other sub-class.
Question 11.
How are the stringed instruments different from the wind instruments ?
Answer:
In wind instruments, air is made to vibrate inside a wooden tube whereas stringed instruments are played by plucking the strings or drawing a bow across the strings.
Question 12.
If you are asked to choose one ofthe musical instruments, which instrument would you choose ? Why?
Answer:
I would choose the flute because it is an amazing instrument. It is one of the smallest instruments available. Listening to the flute music has many benefits. It relaxes the nervous system. It reduces negative effect of anxiety, stress and depression as it soothes the nerves. It aids in better sleep. It obviously makes our mind clearer and helps us to think better.
Question No. (13) : Read the passage given below focussing on the parts that are underlined. Answer any 4 of the questions as directed and write them in the answer booklet. 4 × 2 = 8 M
(i) A tiger was hunting in the forest. He saw a fox.
The fox noticed this. “Now the tiger will kill me,” the fox thought. “But I should do something.” Despite the danger, the fox said, “How dare you kill me !”
On hearing this, the tiger was surprised.
(ii) “Why can’t I kill you?” he asked.
The fox said,”
(iii) God has appointed me as the king of the forest. I have to rule my kingdom.
(iv) You can’t kill me. God will punish you.”
“Is it true?” the tiger asked doubtfully.
“Let’s go through the forest. I’ll show you how everyone is afraid of me,” said the fox.
The tiger agreed. The fox was walking through the forest followed by the tiger.
(v) The animals saw the tiger. They ran away.
“Have you seen how the animals are scared of me ?” the fox said. The tiger thought
(vi) the fox was the most powerful animal. “You’re right,” said the tiger. “You are the king of the forest.”
i) Combine the two sentences using’while’.
ii) Rewrite the sentence beginning with ‘He asked why …………
iii) Rewrite the sentence beginning with “I”
iv) Combine the two sentences using ‘if’.
v) Combine the sentences using ‘no sooner ………….. than’.
vi) Rewrite the sentence beginning with ‘no other’.
Answer:
i) While the tiger was hunting in the forest, he saw a fox. (Or)
While hunting in the forest, the tiger saw a fox. (Or)
The tiger saw a fox while he was hunting in the forest.
ii) He asked why he could not kill him.
He asked why he could not kill the fox.
iii) I was appointed as the king of the forest by God. (Or)
I was appointed by God as the king of the forest.
iv) If you kill me, God will punish you.
v) No sooner had the animals seen the tiger than they ran away. (Or)
No sooner did the animals see the tiger than they ran away.
vi) No other animal was as powerful as the fox. (Or)
No other animal was so powerful as the fox.
![]()
Question No. (14) :
In the text ‘My Childhood’, we have come across how the new teacher insulted Kalam and how Laxmana Sastry changed his mind. Finally, the new teacher realised and apologized for he had done.
Now write a possible conversation between the new teacher and Laxamana Sastry in ten to twelve exchanges. 10 M
You may use the following ideas:
* The new teacher couldn’t digest a Muslim boy sitting beside a Brahmin boy.
* He believed in customs related social status.
* Laxmana Sastry understood the pain of the two boys due to separation.
* Laxmana Sastry believed in equality and brotherhood.
* The new teacher realized and understood the feelings of the two boys.
(OR)
Nowadays family relationships are becoming weak. People have become so selfish that they always think about money and assets. Even members of the family do not show affection to-wards elders.
Prepare a speech script in this context in ten to twelve sentences.
You may use the following ideas.
* Importance of maintaining family relationship.
* Money not as important as family.
* Need to show love and affection towards elders.
* Maintaining good relationship among the members of the family.
Answer:
Possible conversation between Lakshmana Sastry and the teacher:
Lakshmana Sastry : Good morning, sir.
New teacher : Good morning, what can I do for you, sir ?
Lakshmana Sastry : Are you the new teacher ?
Teacher : Yes, I am. I joined this school yesterday only.
Lakshmana Sastry : Ramanatha Sastry is my son.
Teacher : Is there anything wrong with him ?
Lakshmana Sastry : Nothing with him, but I want to talk with you about the yesterday’s incident.
Teacher: What happened yesterday ?
Lakshmana Sastry : You separated Kalam from my son. You asked Kalam to go and sit on the back bench. Both of them are very sad. They are close friends. Why did you do this ?
Teacher : But, you are Brahmins and that boy is a Muslim.
Lakshmana Sastry : Stop talking. You should not spread the poison of social inequality and communal intolerance in the minds of innocent children.
Teacher : But, sir ……………..
Lakshmana Sastry : You must either apologize or quit the school and the island.
Teacher : Yes, sir. I am very sorry. My profound apologies to you, sir. The thing that happened yesterday was an unfortunate one.
Lakshmana Sastry : That’s good. You shouldn’t repeat it in the future.
Teacher : OK., sir
(OR)
Good morning respected Headmaster, beloved teachers and my dear fellow students.
Today 1, xxxx, would like to share my views on the family relations which are becoming weaker nowadays. There are several reasons for this unfortunate development. The most important one, of course, is the change in our lifestyles, values and goals.
Families that spend a lot of time together tend to be closer. And families where the members rarely come face to face with one another have hardly anything that ties them together. In the olden days parents and children used to live and work together. In many families, children pursued the same career as their father. They learned the trade or craft from their parents arid elder siblings. This allowed them to spend more time with their family and that made their relationships stronger.
Now we are living in the age of individualism. Everyone needs their space. This has made us more self centered than our parents or grandparents. This also reflects in our relationships. We no longer believe in making adjustments or compromises. We want everything our way. This attitude creates strain in our relationships.
There is yet another reason that affects family bonds. People are increasingly moving to another city or country in search of better education, jobs or living standards. They leave their family behind. While it is possible to keep in touch through modern communication means, an email or phone call is not equal a face-to-face interaction. The only way to forge better ties with our near and dear ones is to spend more time with them.
Nowadays people have become so selfish that they always think about money and assets. Even members of the family do not show affection towards elders. It pains to say that youngsters todays have neither time nor respect for the elders. Our busy lifestyle gives us no time to spend with them. They feel lonely and neglected. Our elders gave us their precious time, love, care, money, education, etc. If they had not done so, what would have been our situation ? So it is our responsibility to give them back their love and concern.
We must reslize that our biggest strength is our family. Relationships are like potted plants. We need to nourish them. If we don’t give them the love and affection they need, they will wilt. If we need better family relationships, we must be willing to adjust and compromise. We must realize that the only way to win love is to give love. We must remember that the strength of a family, like the strength of an army, is in its loyalty to each other.
Thank you for giving me this opportunity to share my feelings with you.
![]()
Question No. (15) :
On the occasion of International Women’s Day, your school is organizing Mandal Level Sports. Meet for girls of secondary level.
Imagine you are the girls’ representative of your class and draft a notice. Also request the girls of your class to participate in the Sports Meet. 5M
Answer:
|
KAMALA NIKETAN, CHATRAI. We are pleased to inform that ‘Mandal Level Sports Meet’ for girls of secondary level is going to be organised in our school on the occasion of International Women’s Day. The Sports Meet will be held on 06 – 03 – 20xx. The girls are requested to participate in the programme and take active part in Sports Meet and make it a grand success. The girls who are interested to participate in the Sports Meet are asked to give their names to their class teachers. Sd/- Date : 03.03.20xx |
Question No. (16) :
Read the following information about Sachin Tendulkar, the famous Indian cricketer.


Now write a short profile of Sachin Tendulkar in a paragraph based on the above information. 5 M
Answer:
Sachin Tendulkar is an Indian former international cricketer who captained the Indian national team. He is regarded as one of the greatest batsmen in the history of cricket. He was born on 24 April, 1973 at Dadar in Muiribai. His father named Ramesh Tendulkar was a famous Marathi poet and novalist. His mother Rajni worked in the insurance industry.
On 11 December 1988 Sachin represented Mumbai team in Ranji Trophy and scored 100 runs. He was the youngest Indian cricketer to score a century on debut in first-class cricket. He made his Test Debut against Pakistan on 15 November 1989 and One Day International Debut aganist Pakistan on 18 December 1989. He played 200 Test matches and scored 15921 runs. His highest score in Test matches was 248. He got 46 wickets in Test matches. He played 463 ODI and scored 18426 runs. His highest score in ODIs was 200. He got 154 wickets in ODIs.
Sachin Tendulkar received many prestigious awards. He received the Aijuna Award in 1994, the Padma Sri Award in 1999 and the Padma Vibhushan Award in 2008 by the government of India. He was also honoured by the government of India with the India’s highest civilian award the Bharat Ratna in 2014.
Part – B (20 Marks)
Instructions :
- Answer the questions on the question paper itself and attach it to the answer booklet of Part – A.
- Avoid overwriting.
Questions 17 – 21 : Read the following poem.
| Over the mountains, Over the plains, Over the rivers, Here come the trains. |
Thousands of freight cars All rushing on Through day and darkness, Through dusk and dawn. |
| Carrying passengers, Carrying mail, Bringing their precious loads In without fail. |
Over the mountains, Over the plains, Over the rivers, Here come the trains. |
Now answer the questions. Each question has four choices. Choose the correct answer and write (A), (B), (C) or (D) in the brackets given. 5 × 1 = 5 M
Question 17.
The poem is mainly about ……………….
A) travelling in a train
B) the movement of trains
C) the speed of trains
D) the facilities in the train
Answer:
B) the movement of trains
Question 18.
The word ‘freight’ means ………………
A) goods carried from one place to another
B) passengers travelling in a train
C) everything in the train
D) important things in the train
Answer:
A) goods carried from one place to another
Question 19.
‘Through dusk and dawn’ means ……………….
A) in the morning
B) in the evening
C) in daytime
D) all the time
Answer:
D) all the time
Question 20.
The word ‘precious’ means ………………….
A) valuable
B) heavy
C) important
D) comfortable
Answer:
D) comfortable
Question 21.
The mood of the poem is ………………..
A) funny
B) serious
C) gloomy
D) enjoyable
Answer:
D) enjoyable
Questions (22 – 26): In the following passage, five sentences are numbered and each of them has an error. Correct them and rewrite them in the given.space. 5 × 1 = 5 M
An old tiger ran through the rain looking for shelter. (22) He was wet and cold so his cave was far away. (23) While hurried to his shelter he saw an old hut. (24) With a sigh of relief the tiger crawled in the thatched roof and lay down by the door. (25) Except for the sound of the rain was quiet all. Before he could nod off, however, he heard something heavy being dragged inside the hut. (26) This was followed on the voice of a woman.
Answer:
22. He was wet and cold and his cave was far away.
23. While hurrying to his shelter he saw an old hut.
24. With a sigh of relief the tiger crawled under / beneath the thatched roof and lay down by the door.
25. Except for the sound of the rain all was quiet.
26. This was followed by the voice of a woman.
Questions (27 – 31) : Complete the passage choosing the right words from those given below. Each blank is numbered and each blank has four choices (A), (B), (C) and (D). Choose the correct answer and write (A), (B), (C) or (D) in the brackets given. 5 × 1 = 5M
The Olympic Games ……………….. (27) in Olympia in 776 B.C. They took place from time to time ……………… (28) they stopped. At first, they lasted only one day and there was only one race. Later there were ………………. (29) races and contests and the Games lasted several days. People all over Greece ……………….. (30).
In 1894, a French man, Baron De Coubertin thought of starting the Games again and in 1896 the first Modern Olympics took place in Athens in Greece. The Greeks did not do very well, …………. (31) they won a very long race called Marathon.
Question 27.
A) begin
B) begins
C) has begun
D) began
Answer:
D) began
Question 28.
A) until
B) through
C) on
D) to
Answer:
A) until
Question 29.
A) more
B) the more
C) much
D) much more
Answer:
A) more
Question 30.
A) took up
B) took out
C) took part
D)took for
Answer:
C) took part
Question 31.
A) and
B) but
C) since
D) because
Answer:
B) but
Questions (32 – 36) : Read the following passage with focus on the underlined parts. Answer them as directed in the space given. 5 × 1 = 5M
I gave him the can of wine. He poured himself a mug and handed me the can. He drank all of it (32) at one go. He then arranged the belt that was (33) attatched to the trunk carefully on his forehead. So, this was the picture: my father carrying my luggage on his back and me following him with a (34) tiny bag in my hand. We were walking up a narrow hilly road, and neither of us uttered a word as if we were strangers who spoke different languages. I did not know what was going on in his mind. From time, to time it crossed my mind that it was improper for me to let father carry the luggage. I wanted to tell him that I would like to carry the trunk myself, but my guilt and shame did not allow me to do so. This self-consciousness had probably to do with my education, the white-collar job that I had, or quite simply my (35) proud. Somehow, I had the feeling that if I carried the luggage, my father and my people, in fact the (36) hole world would laugh at me and I would be belittled.
Questions :
32. Give the meaning of the underlined expression.
Answer:
all at once/all at one time/continuously/non-stop
33. Give the correct spelling of the underlined word.
Answer:
attached
34. Give the word that is opposite in meaning.
Answer:
huge / very big / large / vast
35. Replace the underlined word with its correct form.
Answer:
pride
36. Replace the underlined word with the suitable word which is pronounced similarly.
Answer:
whole




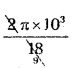 T = \(\frac{\pi}{9}\) × 103 sec
T = \(\frac{\pi}{9}\) × 103 sec