Students can go through AP Inter 2nd Year Chemistry Notes 6th Lesson P-Block Elements will help students in revising the entire concepts quickly.
AP Inter 2nd Year Chemistry Notes 6th Lesson P-Block Elements
Group – 15 Elements
→ N, P, As, Sb mid Bi are Group – 15 elements.
→ General electronic configuration ns2 np3.
→ In group – 15 elements ‘N’ exhibits anamalous properties.
→ Dinitrogen Preparation:
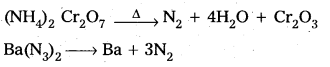
Uses :
- In the manufacture of NH3, other industrial chemicals.
- It is used in cryosurgery.
- To create inert atmosphere.
![]()
→ Ammonia Preparation :
N2 + 3H2 ⇌ 2NH3 + 92.2 KJ
(NH4)2SO4 + 2NaOH → 2NH3 + 2H2O + Na2SO4
(NH4)2CO3 → 2NH3 + H2O + CO2
NH3 is a lewis base and it donates electron pair to form dative bond with metal ions. This results in the formation of complex compound.
Eg : 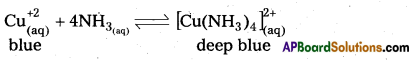
Uses:
- Used in the manufacture of nitrogenous fertilisers. .
- Used as refrigerent. .
- Used in the manufacture of HNO3.
→ Nitric Acid Preparation :
Ostwald’s Process :
![]()
2NO + O2 ⇌ 2NO2
3NO2 + H2O → 2HNO3 + NO
Uses :
- In the manufacture of Nitro glycerine trinitro toluene.
- Used in the pickling of stainless steel.
- Used in etching of metals.
→ Phosphorous:
- Important allotropes of phosphorous are white phosphorous, red phosphorous, black phosphorous.
- White phosphorous reacts with boiled NaOH solution in an inert atmosphere forms phosphine.
P4 + 3NaOH + 3H2O → PH3 + 3Na2H2PO2 - White phosphorous heated at 573K in inert conditions for few days forms red phosphorous.
- Red phosphorous heated in a sealed tube at 803 K to form a-black phosphorous.
- White phosphorous heated at 473K under high pressure to form p-black phosphorous.
![]()
→ Phosphine Preparation :
Ca3P2 + 6H2O → 3Ca(OH)2 + 2PH3
PH4I + KOH → KI + H2O + PH3
Uses:
- A Mixture of Ca3P2 and CaC2 is used in Holme’s signal.This Mixture containing containers are pierced and thrown in the sea, when the gas is evolved bum and serve as a signal.
- The spontaneous combustion of PH3 is the technical use of Holme’s signal.
→ Oxy acids of Phosphorous :
1. Important oxy acids of phosphorous
Hypo Phosphorous acid H3PO2
Ortho Phosphorous acid H3PO3
Pyro Phosphorous acid H4P2O5
Hypo Phosphoric acid H4P2O6
Ortho Phosphoric acid H3PO4
Pyro Phosphoric acid H4P2O7
Meta Phosphoric acid (HPO3)7
Group – 16 Elements
→ O, S, Se, Te, Po are group – 16 elements.
→ General electronic configuration is ns2 np4
→ In group-16 elements ‘O’ exhibits anomalous properties.
→ Dioxygen Preparation:
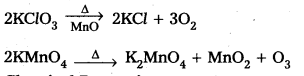
![]()
→ Chemical Properties :
2Ca + O2 → 2CaO
P4 + 5O2 → P4P10
2SO2 + O2 ![]() 2SO3
2SO3
Uses:
- In respiration process.
- In combustion of substances.
- Used in welding of oxy acetylene.
- Oxygen cylinders are widely used in hospitals.
→ Ozone Preparation:
3O2 → 2O3 ∆H° = 142 KJ/mole
Chemical Properties :
PbS + 4O3 → PbSO4 + 4O2
2KI + H2O + O3 → 2KOH + I2 + O2
2Ag + O3 → Ag2O + O2
2Hg + O3 → HgaO + O2 (Tailing of Mercury)
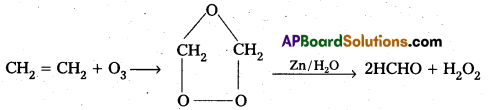
Uses :
- Used as gemicide.
- Used as disinfectant.
- Used for sterilising water.
- Used for bleaching oils, ivory, starch etc.
→ Sulphur:
Important allotropes of sulphur are yellow rhombic (α-sulphur) and Monoclinic (β-sulphur)
![]()
→ Sulphur dioxide (SO2) Preparation:
S + O2 → SO2
4FeS2 + 11O2 → 2Fe2O3 + 8SO2
→ Properties.:
2NaOH + SO2 → Na2SO3 + H2O
SO2 + Cl2 → SO2Cl2
2SO2 + O2 → 2SO3
Uses :
- Used in refining petroleum and sugar.
- Used in bleaching wool and silk.
- Used as an antichlor.
→ Oxo acids of Sulphur:
Important oxy acids of sulphur :
Sulphurous acid H2SO3
Sulphuric acid H2SO4
Peroxo disulphuric acid H2S2O8
Pyro Sulphuric acid (oleum) H2S2O7
Sulphuric acid
Manufacture:
S + O2 → SO2
2SO2 + O2 ![]() 2SO3
2SO3
SO3 + H2SO4 → H2S2O7
H2S2O7 + H2O → 2H2SO4
Uses :
- Used as industrial chemical.
- Used in petroleum refining.
- Used in manufacture of pigments, paints.
- Used in detergent industry.
Group – 17 Elements
→ Fluorine, Chlorine, Bromine, Iodine and Astatine are Group -17 elements.
→ General electronic configuration is ns2 np5
![]()
→ Fluorine exhibits anomalous properties.
Chlorine Preparation :
MnO2 + 4HCl → MnCl2 + Cl2 + 2H2O
4HCl + O2 ![]() 2Cl2 + 2H2O
2Cl2 + 2H2O
Properties :
- 8NH3 + 3Cl2 → 6NH4Cl + N2
NH3 + 3Cl2 → NCl3 + 3HCl - 2NaOH (Cold.dil) + Cl2 → NaCl + NaOCl + H2O
6NaOH (hot.conc) + 3Cl2 → 5NaCl + NaClO3 + 3H2O - Cl2 + H2O → 2HCl + (O)
Uses:
- Used for bleaching wood pulp.
- Used in extraction of gold and platinum.
- Used in manufacture of dyes, drugs etc.
Oxo acids of Chlorine :
Hypochlorous acid HOCl
Chlorous acid HClO2
Chloric acid HClO3
Perchloric acid HClO4
![]()
Inter Halogen Compounds :
The binary diamagnetic compounds of halogens which are formed by the reaction of halogens among themselves are called interhalogen compounds.
E.g.: IF7, ClF3, BrF3, ClF, IF3 etc.
The above examples are binary diamagnetic compounds and formed by combination of halogens only.
Inter halogen compounds are classified into four types.
- AX-Type : Eg : ClF, BrF
- AX3 – Type : Eg : ClF3, IF3
- AX5 – Type : Eg : ClF5, BrF5
- AX7 – Type : Eg : IF7
‘A’ is less electronegative halogen.
‘X’ is more electronegative halogens.
Group – 18 Elements
→ He, Ne, Ar, Kr, Xe, Ru are the Group – 18 elements.
→ General electronic configuration of these elements is ns2 np6 (Except He)
→ Group-18 elements are chemically inert.
→ ‘Xe’ only forms stable compounds with fluorine, oxygen.
Xenon-Fluorine Compounds:
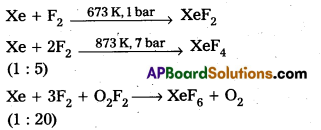
Chemical Properties :
XeF2 + PF5 → [XeF]+ [PF6]–
XeF4 + SbF5 → [XeF3]+ [SbF6]–
XeF6 + 3H2O → XeO3+ 6HF
6XeF4 + 12H2O → 4Xe + 2XeO3 + 24HF + 3O2
XeF6 + H2O → XeOF4 + 2HF
XeF6 + 2H2O → XeO2F2 + 4HF
Compound – Structure
XeF2 – Linear
XeF4 – Square planar
XeF6 – Distorted octahedral
XeOF4 – Square pyramidal
XeO3 – Pyramidal
XeO4 – Tetrahedral
![]()
Uses of Ne:
- Ne is used in discharge tubes and fluorescent bulbs for advertisement display purpose.
- ‘Ne’ – bulbs are used in botanical gardens and in green houses.
Uses of Ar:’
- ‘Ar’ is used to create inert atmosphere in high temperature net allurgical process.
- ‘Ar’ is ued in filling electric bulbs.
Uses of He:
- It is used in filling ballons for meterological observation.
- It is used as Cryogenic agent to provide low temperature.