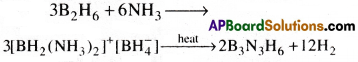Students get through AP Inter 1st Year Chemistry Important Questions 10th Lesson The p-Block Elements – Group 13 which are most likely to be asked in the exam.
AP Inter 1st Year Chemistry Important Questions 10th Lesson The p-Block Elements – Group 13
Very Short Answer Questions
Question 1.
Discuss the pattern of variation in the oxidation states of Boron to Thallium.
Answer:
All the elements of III A group exhibit a common oxidation state of +3. Except boron and aluminium all other elements show +1 oxidation state. This +1 state becomes more and more stable, as we go down the group.
Question 2.
(low do you explain higher stability of BCI3 over TlCl3?
Answer:
Due to the poor shielding effect of the s-electrons of the valency shell by inner electrons, inert pair effect is maximum in Tl. But such effect is minimum in B. Hence BCl3 is more stable when compared to TlCl3.
Question 3.
Why does BF3 behave as a Lewis acid? [AP 22]
Answer:
BF3 is an electron deficient compound because it contains sextet configuration (6 electrons) in the valency shell of Boron. So it can accept a pair of electrons to get octet.
Hence BF3 behaves as Lewis acid.
Question 4.
Is boric acid a protic acid? Explain.
Answer:
Boric acid is a weak mono basic acid. In Boric acid, planar BO3 units are joined by hydrogen bonds. But it acts as a Lewis acid by accepting electrons from a hydroxyl ion. Hence it is not a protic acid.
Question 5.
What happens when boric acid is heated?
Answer:
When Boric acid is heated above 370K it forms meta boric acid. This on further heating forms Boric oxide.
![]()
Question 6.
Describe the shapes of BF3 and BH–4 Assign the hybridization of boron in these species.
Answer:
Shape of BF3 molecule is Trigonal planar. Hybridisation of ‘B’ in BF3 is sp²
Shape of BH–4 molecule is Tetrahedral.
Hybridisation of ‘B’ in BH–4 is sp³.
![]()
Question 7.
Explain why atomic radius of Ga is less than that of ‘Al’. [AP 22]
Answer:
In Gallium, penultimate shell contains 10-d electrons. Due to this 10-d electrons, shielding effect becomes poor on outer most electrons. As a result, the electrons in gallium experience greater force of attraction by the nucleus than in Al. Hence atomic size of Ga(135pm) is slightly less than that of Al( 143pm).
Question 8.
Explain inert pair effect. [TS 17]
Answer:
The reluctance of ‘ns²’ electron pairs to take part in bond formation is called inert pair effect.
Ex: In Group -13, Tl exhibits + l stable oxidation state instead of +3 oxidation state due to inert pair effect.
Question 9.
Write balanced equations for
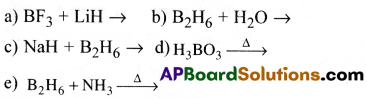
Answer:
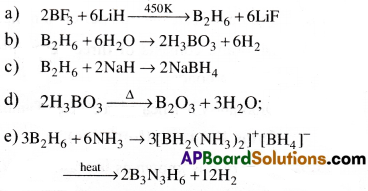
Question 10.
Why is boric acid polymeric?
Answer:
H3BO3 (Boric acid) has layer like structure. In this structure planar BO3 units are joined by hydrogen bonds Hence it forms a polymeric structure.
Question 11.
What is the hybridization of B in diborane and borazine?
Answer:
a) In diborane (B2H6), B undergoes sp³ hybridisation.
b) In borazine (B3N3H6), B undergoes sp² hybridisation.
![]()
Question 12.
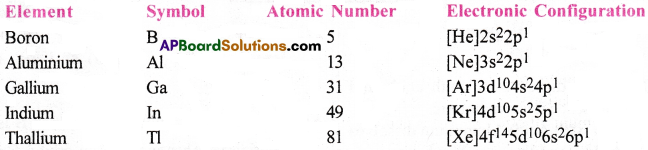
Write the electronic configuration of group-13 elements.
Answer:
The general electronic configuration of group-13 elements is ns²np¹
Question 13.
Give the formula of borazine. What is its common name? [TS 17]
Answer:
The formula of borazine is B3N3H6.
It’s common name is “Inorganic benzene”. Because its structure resembles the structure of benzene ie., Cyclohexagon.
Question 14.
Give the formulae of
a) Borax b) Colemanite
Answer:
a) Borax : Na2B4O7.10H2O
b) Colemanite: Ca2B6O11.5H2O
Question 15.
Give two uses of aluminium.
Answer:
Aluminium is used :
- for making electrical cables, trays, picture frames.
- as a deoxidiser in metallurgy.
- for making alloys.
- as a reducing agent in thermite welding
Question 16.
What happens when a) LiAlH4 and BCl3 mixture in dry ether is warmed and b) Borax is heated with H2SO11?
Answer:
a) When LiAlH4 and BCl3 mixture is warmed in dry ether, diborane is formed
4BCl3 + 3LiAIH4 → 2B2H6 + 3LiCl + 3AlCl3
b) When Borax is heated with H2SO4 then boric acid is formed.
Na2B4O7 + H2SO4 + 5H2O → Na2SO4 + 4H3BO3
![]()
Question 17.
Sketch the structure of Boric acid.
Answer:
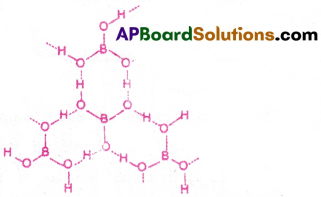
Question 18.
Write the structure of AlCl3 as dimer.
Answer:
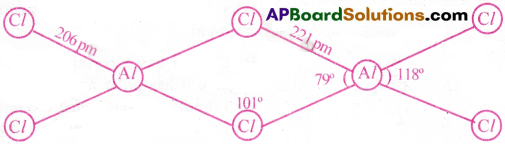
Question 19.
Metal borides (having 10B) are used as protective shield -Why?
Answer:
Boron -10 (10B) has high ability to absorb neutrons. Hence metal borides (having 10B) are used as protective shields and control rods in nuclear industry.
Short Answer Questions
Question 1.
Write reactions to justify amphoteric nature of aluminium.
Answer:
Aluminium dissolves in both acids and alkalies. Hence it is amphoteric.
Reaction with Acid: Aluminium dissolves in dilute HCl and liberates dihydrogen.
2Al + 6HCl → 2AlCl3 + 3H2
Reaction with Base:
Aluminium also dissolves in aqueous alkali and liberates dihydrogen.
2Al + 2NaOH + 6H2O → 2Na + [Al(OH)4]– + 3H2
These reaction indicate the amphoteric nature of aluminium.
Question 2.
What are electron deficient compounds? Is BCl3 an electron deficient species? Explain. [TS 22]
Answer:
The compounds in which the central atom has incomplete octet of electrons in its outermost orbit are called electron deficient compounds.
BCl3 is an electron deficient compound.
In BCl3 molecule, the central atom boron has only six electrons in its valency shell. Therefore BCl3 molecule accepts an electron pair to achieve stable electronic configuration and thus behave as Lewis acid.

Eg: BCl3 molecule easily accepts a lone pair of electrons from ammonia to form
Question 3.
Suggest reason why the B-F bond lengths in BF3 (130pm) and BF–4 (143 pm) differ.
Answer:
BF3 is a planar molecule in which B is sp² hybridised. It has an empty 2p-orbital. F-atom has three lone pairs of electrons in the 2p- obritals. Because of its small size and strong inter electronic repulsions, pπ – pπ back bonding or back donation occurs in which a lone pair is transfered from F to B.

As a result of this back bonding, B-F bond acquires some double bond character. In contrast, in [BF–4] ion, B is sp-’ hybridised and hence does not have an empty p-orbital available to accept the electrons donated by the F atom. Consequently, in B-F is a purely single bond. Since double bonds are shorter than single bonds, the B-F bond length in BF3 is shorter (130 pm) than B-F bond length (143pm) in [BF–4]
Question 4.
B-Cl bond has a bond moment. Explain why BCl3 molecule has zero dipole moment.
Answer:
B-Cl bond has a certain dipole moment because it has polar nature. But BCl3 has zero dipole moment since the molecule is symmetrical (planar triangular) in which net dipole moment (μ) is zero.

Question 5.
Explain the structure of Boric acid.
Answer:
Boric acid contains planar BO3 units which are linked together through hydrogen bonding. Hydrogen atoms act as bridge between two oxygen atoms of different BO3 units. This results in a trigonal planar structure. Boron atom in boric acid is sp² hybridized.
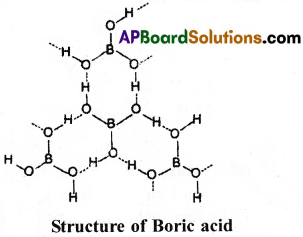
The dotted lines represent hydrogen bonds.
![]()
Question 6.
What happens when
(a) Borax is heated strongly
(b) Boric acid is added to water
(c) Aluminium is heated with dilute NaOH
(d) BF3 is treated with ammonia
(e) Hydrated alumina is treated with aq. NaOH solution.
Answer:
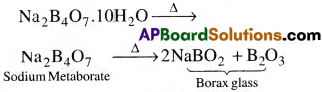
(a) On heating, borax loses water molecules and swells into a white, opaque mass of anhydrous sodium tetraborate.
On further heating, it turns into a transparent liquid, which modifies into glass like material known as borax bead.
(b) Boric acid is slightly soluble in cold water and is readily soluble in hot water.
B(OH)3 + 2H2O → [B(OH)4]– + H3O+
(c) When Aluminium is dissolved in dilute NaOH it liberates hydrogen gas.
2Al + 2NaOH + 6H2O → 2Na+[Al(OH4)]– + 3H2 ↑
(d) When BCl3 is treated with NH3 it forms Ammonium boron trifluoride.
BCl3 + NH3 → [BCl3←NH3] → [BCl3.NH3]
(e) When Hydrated Aluminium is treated with aq.NaOH solution it forms Sodium meta aluminate.
![]()
Question 7.
Give reasons
a) Cone HNO3 can be transported in aluminium container.
b) A mixture of dil. NaOH and aluminium pieces is used to open drain.
c) Aluminium alloys are used to make aircraft body.
d) Aluminium utensils should not be kept in water overnight.
e) Aluminium wire is used to make transmission cables.
Answer:
a) Cone. HNO3 initially reacts with aluminium to form aluminium oxide (Al2O3). This forms a protective coating inside the container. The metal becomes passive and it does not react with the acid any more. Therefore, the acid can be safely stored in aluminium container.
b) NaOH reacts with Al to evolve dihydrogen gas, whose high pressure is enough to open clogged drains.
c) Alloys of aluminium like duralumin are light, tough and resistant to corrosion. Hence they are used in making air craft bodies.
d) Aluminium reacts with water and dissolved O2 to form a thin film of aluminium oxide.
2Al + O2 + H2O → Al2O3 + H2
e) Al metal is not affected by air, moisture. And also due to its good conductivity, it is used to make transmission cables.
Question 8.
Explain why the electronegativity of Ga, In and Tl will not vary very much.
Answer:
The d-electrons present in Ga, In and Tl cannot shield the valence electrons from the nuclear attractions. Hence their valence electrons are attracted strongly by the nucleus. Hence, the electro negativity of Ga, In and Tl will not vary much.
![]()
Question 9.
Explain Borax bead test with a suitable examples. [AP 18, 20][TS 16, 19, 20]
Answer:
Borax bead test:
This test is used for the identification of basic radicals in qualitative analysis.
On heating, borax loses water molecules and swells into a white, opaque mass of anhydrous sodium tetraborate.
On further heating, it turns into a transparent liquid, which modifies into glass like material known as borax bead.
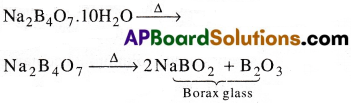
The metaborates of many transition elements have characteristic colours and therefore, borax bead test can be used to identify them in the laboratory.
Ex: When borax is heated in a bunsen burner flame with cobalt oxide on a loop of platinum wire, a blue coloured Co(BO2)2 bead is fonned.

Question 10.
Explain the structure of diborane. [AP,TS 15,16,17,18,19,19]
Answer:
I) Structure of diborane:
The molecular formula of diborane is B2H6. Electron diffraction studies have shown that diborane contains two coplanar BH2 groups.
Its structure can be represented as follows:
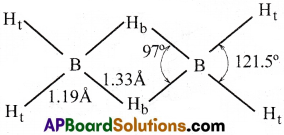
II) Structure of diborane:
- Diborane contains 2 coplanar BH2 groups.
- The four hydrogen atoms located at the ends as shown in the above figure are known as terminal hydrogen atoms (Ht).
- The middle positioned 2 hydrogen atoms are called bridge hydrogens (Hb).
- These two bridge hydrogens lie in a plane perpendicular to the plane of the BH2 groups.
- One of the bridge hydrogens lies above the plane and the other lies below the plane.
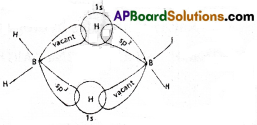
- In diborane, Boron undergoes sp³ hybridisation to form 4 sp³ hybrid orbitals.
- Out of 4 orbitals, 3 orbitals contain one electron each and the fourth orbital is vacant.
- The 2 sp³ hybrid orbitals of each Boron atom fonns 2 sigma bonds with 2 H atoms.
- The bridge between two Boron atoms is formed due to overlap of vacant sp³ orbital of one Boron, ‘s’ orbital of hydrogen and sp³ orbital of another Boron containing one electron.
- Hence this hydrogen bridge is considered as ‘three centered two electron bond’ (or) Banana bond (or) Tau bond.
Question 11.
Explain the reactions of aluminium with acids.
Answer:
1) Aluminium reacts with dilute or cone. HCl and liberates H2.
2Al + 6HCl → 2AlCl3 + 3H2
2) Al reacts slowly with dil. H2SO4 in cold condition but reacts fast in hot condition.
2Al + 3H2SO4 → Al2(SO4)3 + 3H2
3) Al reacts with cone. H2SO4 and liberates SO2.
2Al + 6H2SO4 → Al2(SO4)2 + 6H2O + 3SO2
4) Al reacts with very dil. HNO3 and gives NH4NO3.
8Al + 30HNO3 → 8Al(NO3)3 + 3NH4NO3 + 9H2O
5) Concentrated nitric acid renders the aluminium passive.
Question 12.
Write a short note on the anomalous behaviour of boron in the group-13.
Answer:
Anomalous of behaviour of boron is due to
a) Its small size.
b) The difference in the penultimate shell configuration.
c) High first ionization potential.
d) Absence of d-orbitals.
Anomalous properties of Boron:
- Boron is a non-metal. ‘Al’ is amphoteric. Ga, In and Tl are metals.
- B2O3 is an acidic oxide. The trioxides of others are either amphoteric or basic in nature.
- B(OH)3 is an acid while the hydroxides of other elements are either amphoteric or basic.
- Boron always forms covalent compounds, while the others form ionic compounds.
- Boron has a maximum covalency of 4 only. But others exhibit a maximum covalency of 6.
- Boron shows diagonal relation ship with silicon. Such a relationship is not shown by other elements.
- Boron does not displace hydrogen from acids while others displace hydrogen from acids under suitable conditions.
- Boron can form stable hydrides while the others cannot form stable hydrides.
- Simple borates and silicates can polymerise readily forming polyacids while others do not form such polymers.
Question 13.
Aluminium reacts with dil.HNO3 but not with cone. HNO3 – explain.
Answer:
Dilute HNO3- reacts with aluminium slowly and forms aluminium nitrate and ammonium nitrate.
8Al + 30HNO3 → 8Al(NO3)3 + 3NH4NO3 + 9H2O
Aluminium does not react with cone. HNO3.
Reasons:
Aluminium is passive towards cone. HNO3 due to the formation of thin film of Al2O3 layer on the surface.
Because of this passivity between Al and cone. HNO3, cone. HNO3 is transported in Aluminium containers.
Question 14.
Give two methods of preparation of diborane.
Answer:
1) Diborane can be prepared by treating boron trifluoride with LiAlH4 in diethyl ether.
4BF3 + 3LiAlH4 → 2B2H6 + 3LiF + 3AlF3
2) In the laboratory, it is conveniently prepared by the oxidation of sodium borohydride with iodine.
2NaBH4 + I2 → B2H6 + 2NaI + H2
3) On large scale, it is prepared by reaction of BF3 with sodium hydride.
![]()
![]()
Question 15.
How does diborane react with [AP 19]
a) H2O
b) CO
c) N(CH3)3
d) NH3
Answer:
a) Diborane reacts with water to give Boric acid.
B2H6(g) + 6H2O(l) → 2B(OH)3(aq) + 6H2(g)
b) Diborane reacts with carbon monoxide to give Boran Carbonyl.
B2H6 + 2CO → 2BH3.CO
c) Diborane reacts with trimethyl ammine to give an adduct.
B2H6 + 2N(CH3)3 → 2BH3N(CH3)3
d) Dibroane reacts with ammonia it forms an addition product with asymmetric cleavage which on heating forms borazine which is known as inorganic benzene.
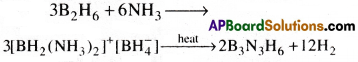
Question 16.
Al2O3 is amphoteric -explain with suitable reactions.
Answer:
Al2O3 reacts with both acids and bases. While reacting with acids it behaves like base. In the reaction with bases it behaves like acid. So it is amphoteric.
Reaction with acid:
Al2O3 + 6HCl → 2AlCl3 + 3H2O
Reaction with base:
Al2O3 + 2NaOH → 2NaAlO2 + H2O
Question 17.
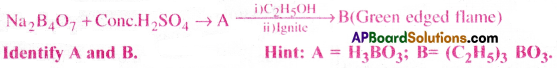
Answer:
When borax is heated with conc.H2SO4 boric acid is formed.
A mixture of ethyl alcohol with boric acid burns with green edged flame due to the formation of ethylborate.
Long Answer Questions
Question 1.
How are borax and boric acid prepared? Explain the action of heat on them.
Answer:
Borax (Sodium tetraborate-Na2B4O7) is an important compound of boron.
I) Preparation of Borax:
1) From tincal:
Borax is obtained from tincal by boiling it with water. The solution is filtered to remove in soluble impurities of sand, clay etc. The solution is concentrated till the crystals of borax separate out.
2) From Colemanite: Colemanite ore (Ca2B6O11). When boiled with sodium carbonate solution, borax is formed along with sodium metaborate.
![]()
3) From boric acid:
Small quantities of borax are obtained by neutralizing boric acid solution with soda ash.
4H3BO3 + Na2CO3 → Na2B4O7 + 6H2O + CO2
On cooling, crystals of Na2B4O7.10H2O separate out.
II) Preparation of Orthoboric acid(H3BO3):
1) Orthoboric acid is usually prepared by acidifying an aqueous solution of borax.
Na2B4O7 + 2HCl + 5H2O → 2NaCl + 4B(OH)3
2) Orthoboric acid is also prepared by the hydrolysis of boron compounds[halides, hydrides etc]
BCl3 + 3H2O → H3BO3 + 3HCl
BN + 3H2O → H3BO3 + NH3↑
III) Action of heat on Borax:
1) On heating borax swells into a white, opaque mass of anhydrous sodium tetraborate.
When it is fused, borax glass is obtained. This contains metaborate and B2O3.
![]()
2) When borax is heated with cone. H2SO4 boric acid is formed.
Na2B4O7 + H2SO4 + 5H2O → Na2SO4 + 4H3BO3
IV) Action of heat on boric acid:
Boric acid on heating first gives metaboric acid at low temperature but at red hot condition forms boron trioxide.
![]()
![]()
Question 2.
How is diborane (B2H6)prepared? Explain its structure.
Answer:
I) Structure of diborane:
The molecular formula of diborane is B2H6. Electron diffraction studies have shown that diborane contains two coplanar BH2 groups.
Its structure can be represented as follows:

II) Structure of diborane:
- Diborane contains 2 coplanar BH2 groups.
- The four hydrogen atoms located at the ends as shown in the above figure are known as terminal hydrogen atoms (Ht).
- The middle positioned 2 hydrogen atoms are called bridge hydrogens (Hb).
- These two bridge hydrogens lie in a plane perpendicular to the plane of the BH2 groups.
- One of the bridge hydrogens lies above the plane and the other lies below the plane.
- In diborane, Boron undergoes sp³ hybridisation to form 4 sp³ hybrid orbitals.
- Out of 4 orbitals, 3 orbitals contain one electron each and the fourth orbital is vacant.
- The 2 sp³ hybrid orbitals of each Boron atom fonns 2 sigma bonds with 2 H atoms.
- The bridge between two Boron atoms is formed due to overlap of vacant sp³ orbital of one Boron, ‘s’ orbital of hydrogen and sp³ orbital of another Boron containing one electron.
- Hence this hydrogen bridge is considered as ‘three centered two electron bond’ (or) Banana bond (or) Tau bond.

1) Diborane can be prepared by treating boron trifluoride with LiAlH4 in diethyl ether.
4BF3 + 3LiAlH4 → 2B2H6 + 3LiF + 3AlF3
2) In the laboratory, it is conveniently prepared by the oxidation of sodium borohydride with iodine.
2NaBH4 + I2 → B2H6 + 2NaI + H2
3) On large scale, it is prepared by reaction of BF3 with sodium hydride.
![]()
![]()
Question 3.
Write any two methods of preparation of diborane. [AP 18]
How does it react with a) Carbon monoxide and b) Ammonia?
Answer:
1) Diborane can be prepared by treating boron trifluoride with LiAlH4 in diethyl ether.
4BF3 + 3LiAlH4 → 2B2H6 + 3LiF + 3AlF3
2) In the laboratory, it is conveniently prepared by the oxidation of sodium borohydride with iodine.
2NaBH4 + I2 → B2H6 + 2NaI + H2
3) On large scale, it is prepared by reaction of BF3 with sodium hydride.
![]()
4) Diborane reacts with water to give Boric acid.
B2H6(g) + 6H2O(l) → 2B(OH)3(aq) + 6H2(g)
5) Diborane reacts with carbon monoxide to give Boran Carbonyl.
B2H6 + 2CO → 2BH3.CO
6) Diborane reacts with trimethyl ammine to give an adduct.
B2H6 + 2N(CH3)3 → 2BH3N(CH3)3
7) Dibroane reacts with ammonia it forms an addition product with asymmetric cleavage which on heating forms borazine which is known as inorganic benzene.