AP State Syllabus AP Board 8th Class Physical Science Solutions Chapter 3 Matter Around Us Textbook Questions and Answers.
AP State Syllabus 8th Class Physical Science Solutions 3rd Lesson Matter Around Us
8th Class Physical Science 3rd Lesson Matter Around Us Textbook Questions and Answers
Improve Your Learning
Question 1.
Describe an activity which provides the evidence for
a) the motion of particles
b) attraction between particles
c) inter-particle space
Answer:
a) An activity which provides the evidence for the motion of particles:
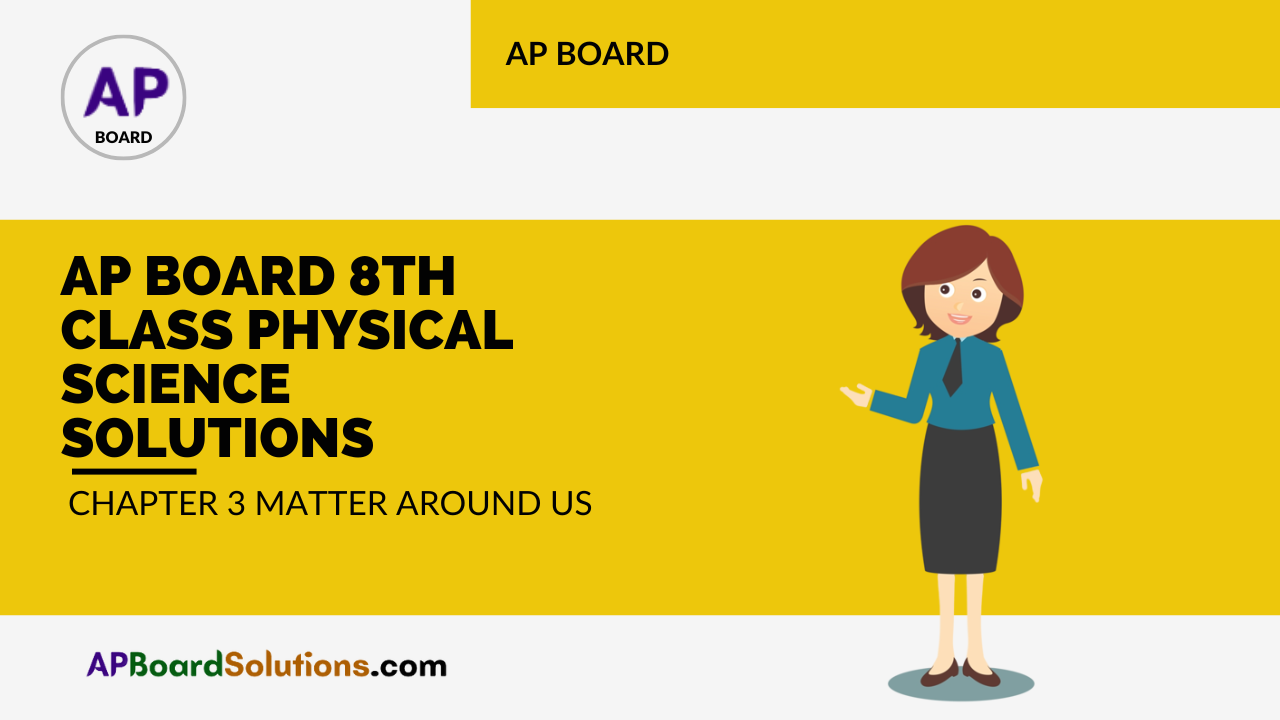
Materials required: Two 250 ml round bottom flasks, red or blue ink, dropper, Potassium permanganate (KMnO4) solution.
Procedure:
- Take two 250 ml round bottomed flasks and fill them with water.
- Use a dropper and put a few drops of blue or red ink slowly along the sides of first flask.
- Use a dropper and put one or two drops of KMnO4 solution along the sides of second flask.
- We observe that in the first flask the ink drops slowly diffusing and mix with water, so as to change the colour of water to red/blue.
- In the second flask we observe that the KMnO4 solution diffuses fast in the water and the colour of water changes.
- This activity provides an evidence for the motion of particles.
![]()
b) An activity which provides the evidence for the attraction between particles:
- Open a water tap and allow the water to reach the ground.
- Now try to break the stream of water with your finger.
- We can break the stream momentarily, but not permanently.
- The reason behind the stream of water remaining together is the force of attraction between the water particles.
- Now try to break a piece of iron nail with your hands.
- We cannot break it, because the force of attraction between the particles of nail is very high.
- Do the same with a piece of chalk. We can break it easily, due to the weak attraction forces among the particles of piece of chalk.
- From the above, we can say that the particles of matter have attractive forces among them to keep the particles together.
- These forces of attraction are not equally strong in all the forms of matter.
c) An activity which provides the evidence for the inter-particle space:
Materials required: Beaker, water, salt, spoon and a glass rod.
Procedure:
- Take a graduated beaker and fill it with some water and mark the level of water.
- Add some salt and stir it thoroughly with a glass rod.
- Observe the change in water level. There will be no change in it.

- Add some more salt and stir it again.
- Observe the change in water level. There will be no change.
- Continue this process till you see some salt remains undissolved in water.
- From this activity, we can say that solid and liquid particles have some space between them.
- The solid particles enter into the space between the liquid particles on dissolving solid in liquid.
- This process continues till all the space in liquid particles is occupied by solid particles.
- After completion of this occupation, the solid particles, as there is no space to occupy remain undissolved.
![]()
Question 2.
Name the characteristics of matter that are demonstrated by diffusion.
Answer:
The characteristics of matter that are demonstrated by diffusion:
- Matter is made up of tiny particles.
- Particles of matter have space between them.
- Particles of solids and liquids diffuse into liquids.
- Particles of gases diffuse into gases.
- Rate of diffusion of gases is higher than that of liquids or solids.
- Particles of solids occupy the space between the particles of liquids on addition of a solid to a liquid.
- Particles of matter move continuously in liquids and gases.
Question 3.
“When sugar is dissolved in water, there is no increase in volume.” Is it true or false? Comment on the statement keeping in mind the amount of sugar, amount of water, etc.
Answer:
This statement is true.
Reason: When sugar is dissolved in water, the particles of sugar occupy the space between the particles of water. Hence there is no change in the volume of water.
Conditions for observing the change in volume of water:
If we go on adding sugar without increasing the quantity of water, there will be no space between water particles, to be occupied by sugar particles. Hence the sugar remains undissolved.
Question 4.
Is there any change in mass when a substance changes its state? Explain with example.
Answer:
- Take a beaker and fill it with ice cubes.
- Find the mass (m1) of the beaker along with ice cubes using a spring balance.
- Keep the beaker at rest for some time, till the ice changes into water.
- Then find the mass (m2) of the beaker along with water.
- We observe that m1 = m2.
- We conclude that the mass never changes when a substance changes its state.
Question 5.
Do all substances change from solid to liquid and liquid to gas on heating ? Explain. Ans. All substances cannot change from solid to liquid and liquid to gas on heating.
e.g.:
- When wood is heated, it cannot change its state from solid to liquid, but the form of substance changes.
- Mercury/blood.
Question 6.
Define the following terms:
a) Melting point
b) Boiling point
c) Evaporation
Answer:
a) Melting point: The temperature at which a solid melts to become a liquid is called “melting point”.
b) Boiling point: The temperature at which a liquid starts boiling at the atmospheric pressure is known as its “boiling point”.
c) Evaporation : The phenomena of change of a liquid into vapours at any temperature below its boiling point is called “evaporation”.
![]()
Question 7.
Correct the following statements.
a) Water boils at 100°C under atmospheric pressure.
Answer:
This is a correct statement.
b) A liquid evaporates above its boiling point.
Answer:
This statement is incorrect.
Reason: Evaporation takes place at any temperature below its boiling point.
c) Solids have the largest inter-particle space.
Answer:
This statement is incorrect.
Correct Statement: The inter-particle space of solids is minimum.
Explanation: The force of attraction between the particles is also very high due to less inter-particle space. Hence solids have definite shape and a fixed volume.
d) Gases have the strongest inter-particle forces.
Answer:
This statement is incorrect.
Correct Statement: Gases have weakest inter-particle forces.
Explanation: The inter-particle space for a gas is maximum. Hence the inter-particle forces are also weak. Due to this gases have no definite shape or fixed volume. They have to be stored in a closed container always.
Question 8.
Why do we prefer to sip hot tea with a saucer rather than a cup ?
Answer:
- The surface area of saucer is more than the surface area of a cup.
- We know that the rate of evaporation increases with an increase of surface area.
- The hot tea particles can easily escape from the saucer than a cup.
- Hence hot tea becomes cold easily in a saucer than a cup.
- So we prefer to sip hot tea with a saucer rather than a cup.
![]()
Question 9.
When water solidifies to ice then heat is
a) Liberated
b) Absorbed
c) No change
d) Depending upon the condition heat may absorbed or liberated.
Answer:
a) Liberated
Question 10.
Convert the following temperatures to the Celsius scale.
a) 283 K b) 570 K
Answer:
a) We know that 273 K = 0°C
283 K = 283 – 273 = 10°C
∴ 283 K = 10°C
b) We know that 273 K = 0°C
570 K = 570 – 273 = 297
∴ 570 K = 297°C
Question 11.
Convert the following temperatures to the Kelvin scale,
a) 27°C b) 367°C
Answer:
a) 27°C = 27 + 273 = 300 K
b) 367°C = 367 + 273 = 640 K
Question 12.
Fill in the blanks.
a) Matter changes from one state to another either raising the …………… or lowering the ……………..
b) A change in which a solid on heating directly changes into vapour state is called …………….
Answer:
a) temperature, temperature
b) sublimation
Question 13.
Match the following.
a) Conversion of liquid into gas ( ) (i) gas
b) Non – compressible ( ) (ii) solid
c) Maximum expansion ( ) (iii) particle
d) Constituents of matter ( ) (iv) evaporation
Answer:
a) iv
b) ii
c) i
d) iii
![]()
Question 14.
How can we smell perfume sitting several meters away from the source?
Answer:
- We know that the particles of gas are highly mobile in the air.
- The particles of perfume vapours also move in air for several meters.
- Hence we can smell perfume, sitting several meters away from the source.
Question 15.
Steam produces more severe burns than boiling water. Think why?
Answer:
- Steam particles have more energy than the particles of boiling water.
- This is because particles in water vapour have absorbed additional energy in the form of latent heat of vaporization.
- So, steam produces more severe burns than boiling water.
Question 16.
Make a model to explain the structure of particles in solids, liquids and gases.
Answer:
Students have to prepare their own models.
Question 17.
How do you appreciate sweating mechanism of human body to control the temperature of the body?
Answer:
- When we do some physical exercise or hard work, sweating is observed on the body.
- The sweat evaporates from the surface of our body by absorbing the heat from your body.
- Thus the particles of liquid absorb energy from our body and escapes to the surroundings.
- Due to this we feel cool.
Different shaped containers, Beaker, Measuring jar, Conical flask, Round bottomed flask, Test tubes, CNG related Pictures, 50 ml syringe, Incense stick, Scent bottle, Potassium permanganate, Copper sulphate, Long glass tube with scale, Liquid Ammonia, Hydrochloric acid, Cotton, Two rubber corks, Two tongs, Dropper, Water, Salt, Thermometer, Spirit lamp, China dish.
8th Class Physical Science 3rd Lesson Matter Around Us InText Questions and Answers
8th Class Physical Science Textbook Page No. 31
Question 1.
Is there any substance which can be found in three states like water?
Answer:
Yes. The substance which can be found in three states like water is wax.
![]()
Question 2.
What are the properties that lead us to consider petrol or milk as liquids?
Answer:
Petrol and milk are considered as liquids because they have no fixed shape. But they get the shape of the container in which they have been poured.
Question 3.
Do solids have definite shape and fixed volume?
Answer:
Yes, solids have definite shape and fixed volume.
8th Class Physical Science Textbook Page No. 32
Question 4.
What is the shape of the water in different containers?
Answer:
Water gets the shape of containers in which it has been poured.
Question 5.
Is it same in all cases or different?
Answer:
Yes, it is same in all cases.
Question 6.
What shape does water take if it spills on the floor?
Answer:
Water spreads on floor if it spills on the floor.
Question 7.
Are the levels of water and milk same?
Answer:
Yes, the levels of water and milk are same.
Question 8.
Can you guess the volume of oil?
Answer:
Volume of the oil is equal to 50 ml.
![]()
Question 9.
What does a fluid mean?
Answer:
Fluid is a substance which can flow easily.
8th Class Physical Science Textbook Page No. 33
Question 10.
Does CNG have a fixed volume?
Answer:
No, CNG does not have a fixed volume.
Question 11.
Does CNG have a definite shape?
Answer:
No, CNG doesn’t have a fixed shape. Its shape is depending on the containers.
8th Class Physical Science Textbook Page No. 34
Question 12.
Does the smell from burning incense stick and deodorant spray reach someone on the other end at the same time?
Answer:
The smell from deodorant spray reaches someone on the other end faster than the smell from incense stick.
8th Class Physical Science Textbook Page No. 36
Question 13.
Why do gases diffuse faster than solids and liquids?
Answer:
The molecules in the gas are far from each other i.e., the inter molecular space is very high than in solids and liquids. Hence they have less attractive power. So, gases diffuse faster than solids and liquids.
Question 14.
When does water change into ice and then into vapour?
Answer:
Water when cooled in a refrigerator, it becomes ice. (Decreasing the temperature).
8th Class Physical Science Textbook Page No. 39
![]()
Question 15.
What type of changes occur inside the matter during a change of state?
Answer:
Inside the matter, we observe the increase/decrease in volume during the change of state.
Question 16.
How does this change of state take place?
Answer:
Change of state takes place by change in temperature.
Question 17.
What does happen to the particles of matter during a change of state?
Answer:
During a change of state, the kinetic energy of particles may increase/decrease, resulting in the increase/decrease of inter molecular attraction.
8th Class Physical Science Textbook Page No. 41
Question 18.
What will does happe when we apply pressure and compress a gas enclosed in a cylinder?
Answer:
When pressure is applied and compressed the volume of a gas in a cylinder decreases. (According to Boyle’s law).
Question 19.
Will the particles come closer?
Answer:
Yes, the inter molecular space between the particles decreases and they come close.
Question 20.
Do you think that increasing or decreasing the pressure can change the state of matter?
Answer:
Change in pressure can change the state of matter.
Question 21.
Can we liquify gases by applying pressure or reducing temperature?
Answer:
Gases can be liquefied by cooling below its critical temperature. Hence, change in temperature and pressure causes the gases to liquefy.
Question 22.
Do we always need to supply heat or change the pressure for changing the state of matter?
Answer:
It is required. But in some cases like natural evaporation of water, it is not necessary.
Question 23.
Can the change of state from liquid to vapour take place without the liquid reaching its boiling point?
Answer:
Yes, it is possible in case of drying the wet clothes. In the process, water direly changes into vapour form without reaching its boiling point.
![]()
Question 24.
Can you give few more examples for evaporation?
Answer:
Evaporation of iodine, drying up of wet body, etc.
Question 25.
What could be the reasons for this type of changes in states?
Answer:
In liquid s, the particles at the surface possess higher energy than particles in the bulk of watt.. The particles on the surface are able to break away from the force of attraction of other particles and change into vapour state.
Think & Discuss
8th Class Physical Science Textbook Page No. 34
Question 1.
Rubber band Activity:
a) Let us stretch a rubber band. Is there a change in its shape?
b) Is rubber band solid or liquid? Why?
Answer:
a) When rubber band is stretched it changes its shape.
b) It is a solid. If stretching is stopped, the rubber band regains its shape. If the stretching is too much, the rubber band permanently loses its shape.
Reason : Rubber band is a solid, but the nature of particles by which the rubber band is made is responsible for the above phenomena.
Question 2.
Powdered salt Activity:
a) Which shape does the powdered salt take?
b) Can you say that salt is a liquid on the basis of change in its shape? Justify your answer. Take a sponge. Observe its shape.
Answer:
a) Powdered salt takes the shape of the container, b) It is a solid.
Justification:
- Change in shape or state means a complete change in the arrangement of particles.
- Powdered salt is a composition of tiny particles, which do not change their shape.
![]()
Question 3.
Sponge Activity:
Can you compress it? Is it a soild? Why? Think. Is anything coming out from the sponge when it is compressed.
Answer:
- Sponge can be compressed.
- It is a solid.
Justification :
- The inter-particle space is slightly more than a rigid body.
- So that it can be compressed.
Question 4.
Why can’t you able compress a wooden block ? (or) Why aren’t you able to compress a wooden block ?
Answer:
- The inter-particle space is very less in a wooden block.
- So we cannot compress it at ordinary conditions.
8th Class Physical Science Textbook Page No. 42
Question 5.
Why do we wear cotton clothes in summer?
Answer:
- More sweat is produced from our body during summer due to excess of external temperature.
- We feel cool when the sweat is evaporated.
- On wearing cotton clothes, the clothes absorb sweat from our body. So, we feel cool.
- Other clothes like silk, polyester, etc. cannot absorb sweat.
- Hence we wear cotton clothes in summer.
Question 6.
Why do we see water droplets on outer surface of a glass containing ice-cold water?
Answer:
- Ice-cold water in the glass cools its surface.
- Air around the glass contains water vapour which is warmer than the surface of the glass.
- Due to the cold glass, air close to its surface will also become cooler.
- This changes the water vapour in the air around the surface of the glass into water.
- This water forms small drops on the outer surface of the glass.
Question 7.
Why do pigs toil in the mud during hot summer?
Answer:
- Generally, the temperature of body is controlled through the sweating evaporation.
- In the case of pigs, these have less sweat pores on their skin surfaces. So sweating evaporation process does not take place.
- Because of this type of arrangement in pigs, they toil in the mud ponds more time during hot summer.
8th Class Physical Science 3rd Lesson Matter Around Us Activities
Activity – 1
![]()
Identifying the shape and volume of liquids
Question 1.
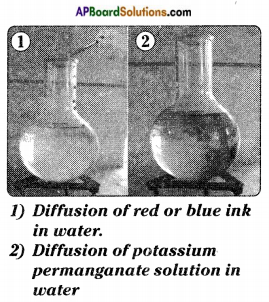
(a) Prove that the liquids have no fixed shape.
Answer:
- Collect transparent containers of different shapes.
- Take some water using the measuring jar.
- Pour the water in one of the containers.
- Observe the shape of water.
- Pour the same water in different containers and observe the shape of water.
- We observe that the water (liquid) takes the shape of container.
- We can say that liquids have no fixed shape.
(b) Prove that the liquids have fixed volume.
Answer:
- Take 50 ml of water with a measuring jar.
- Pour this water in a transparent beaker.
- Mark the level of water on the beaker and throw the water out.
- Now measure 50 ml of milk, and pour it in the same beaker.
- Mark the level of milk on it, and remove milk from the beaker.
- Now take 50 ml of oil and pour it in the same beaker.
- Mark the level of oil on it, and remove the oil from the beaker.
- We observe that the mark on the beaker is same for water, milk and oil.
- This proves that the liquids have a fixed volume.
Activity – 2
Question 2.
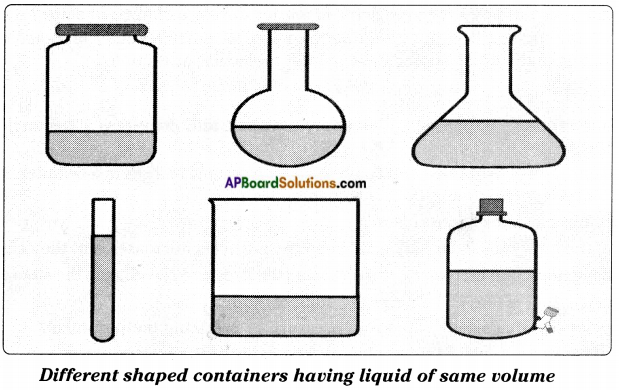
Do the gases have a definite shape and a fixed volume?
Answer:
CNG (Compressed Natural Gas) is stored in a tank. In vehicles CNG is stored in cylinders. We observe that CNG and all other gases neither have a fixed shape nor fixed volume.
Activity – 3
![]()
Observing the compressibility of different materials
Question 3.
Prove that the gases are highly compressible than liquids and solids.
Answer:
- Take a 50 ml syringe.
- Draw the piston to suck in air.

- Place your finger on the nozzle and press.
- Observe depth of piston moved into syringe and note the height of air column.
- We feel hard to press the piston after certain height.
- Here the air in the syringe is compressed.
- Now fill water in the syringe and do the same experiment.
- Note the height of water column when you feel hard to press the piston.
- Here the height of water column is more than the height of air column.
- Now take a piece of wood or iron and press it with your thumb.
- We cannot find any observable change in its volume.
- From the above observations, we can say that gases are highly compressible than liquids and solids.
Activity – 4
Observing the diffusion of gases
Question 4.
Describe an activity to observe the diffusion of gases.
Answer:
- Ask your friend to hold an unlit incense stick and stand in one corner of the room.
- Then you go and stand in the other corner.
- We cannot observe any smell (This depends on the type of incense stick).
- Now ask your friend to light the incense stick.
- After a few seconds, we can observe the smell of incense stick.
- The scent in the vapour form and smoke mixes with air and moves across the room and reaches our nose.
- This activity proves that the gases can diffuse.
Activity – 5
![]()
Observing the diffusion of liquids
Question 5.
Write an activity which shows the diffusion of liquids.
Answer:
Take 250 ml round bottomed flask with 2/3rd water in it. Use a dropper and put a few drops of blue or red ink or Potassium permanganate (KMnO4) solution slowly along the sides of the flask.

We can observe that liquids also diffuse into each other like gases.
Activity – 6
Observing the diffusion of particles of solids into liquids
Question 6.
Prove that the particles of solids diffuse into liquids.
Answer:
- Take a beaker full of water.
- Add a few crystals of potassium permanganate to it.
- We observe that the diffusion of potassium permanganate crystal into water and the colour of water changes.
- Repeat the experiment with crystals of copper sulphate.
- Here also we observe the diffusion of CuSO4 crystals into water and the colour of water changes.
- From the above observations, we found that the particles of solids diffuse into liquids.
Lab Activity Diffusion of two gases
Question 7.
Describe an experiment to measure the speed of diffusion of two gases.
Answer:
Aim: To observe the speed of diffusion of two gases.
Materials required : Long glass tube with scale, liquid ammonia, Hydrochloric acid (HC/), pieces of cotton, two rubber corks and two tongs.

Procedure:
- Take a one meter long narrow glass tube.
- Take two pieces of cotton wool.
- Soak one piece of cotton wool in HCl solution.
- Soak another piece of cotton wool in NH3 solution.
- Insert them separately at the two ends of the tube with the help of tongs.
- Close the ends of the glass tube with rubber cork and observe.
- The HCl gives off hydrogen chloride gas and ammonia solution gives off ammonia gas.
Observation:
- Both gases react together to form a white ring in the tube due to formation of ammonium chloride.
- Measure the distance of white ring from two ends of the glass tube.
Explanation:
We can observe that the ammonia gas travelled faster. So that the distance of white ring is more from ammonia end than hydrochloric acid end.
![]()
Activity – 7
Question 8.
How small are the particles of matter?
Answer:
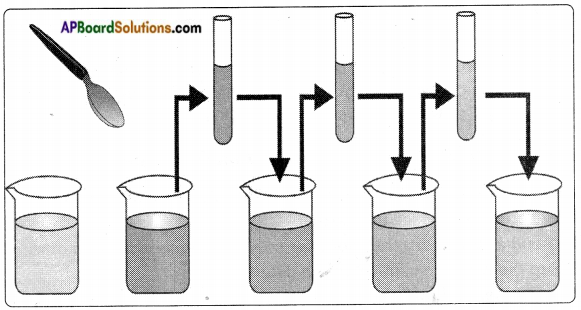
- Take a beaker with water. Mark the level of water.
- Add 1 or 2 crystals of potassium permanganate and dissolve in water.
- Colour of water changes to light violet.
- Now take out approximately 10 ml of this solution and add it to 90 ml of clear water in another beaker.
- Now the colour of water will be lighter than in the previous one.
- Again take out 10 ml of this solution and add it to another 90 ml of clear water.
- Carry out this process 4, 5 times and observe the changes in colour of the solution.
- We observe the water in last beaker also changed to light violet.
- From this activity we conclude that both solids and liquids are made up of tiny particles.
Activity – 8
There exists space between particles
Question 9.
Write an activity which shows the space between particles.
Answer:

Procedure: Take a graduated beaker and fill it with some water and mark the water level. Add some salt and stir it thoroughly with a glass rod. Observe if there is any change in water level. Add some more salt and stir it again.
Observations: From the activity we can conclude that both solid and liquid particles have some space between them. Hence the solid particles enter into the space between the liquid particles on dissolving solid in liquid. So there is no change in the water level.
Activity – 9
Observing the force of attraction between the particles of matter
Question 10.
Write an activity which provides an evidence for the force of attraction between the particles of the matter.
Answer:
Procedure:
Open a water tap and allow the water to reach the ground. Now try to break the stream of water with our finger. But we cannot break the stream permanently.
We cannot also break a piece of iron nail with our hands. But we can break a piece of chalk with our hands.
Observations: From the above observations we can say that particles of the matter have forces acting between them that keeps the particles together.
It is also clear that this force is not equally strong and different in different forms of matter.
![]()
Activity – 10
Effect of temperature on change of state
Question 11.
Write an activity to know the effect of temperature on change of state of matter.
Answer:
- Take about 100 g of ice in a beaker.
- Suspend a laboratory thermometer so that its bulb is in contact with the ice.
- Set up the beaker as shown in the figure.
- Note the temperature.
- Now heat the beaker slowly.
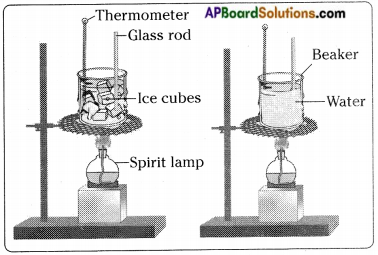
- Record the change in temperature after every 30 seconds.
- Let the ice melt completely.
- Now place a glass rod in the beaker and continue heating till water starts boiling.
- After some time all the water will get vapourised.
- From this we understand that substances around us change state from solid to liquid and from liquid v to gas on application of heat.
- There are some substances which can change directly from solid state to gaseous state and vice versa without changing into the liquid state.
Activity – 11
Effect of surface area, humidity and wind speed on evaporation
Question 12.
(a) Explain the effect of surface area on the rate of evaporation.
Answer:
Rate of evaporation increases with an increase of surface area.
Explanation:
- During evaporation process, the particles escape from the surface of liquid.
- The increase in the surface area provides more scope for particles to escape from the surface.
- Hence it leads to increase the rate of evaporation.
Ex: Water in a China dish evaporates faster than the water in test tube.
(b) Explain the effect of humidity on the rate of evaporation.
Answer:
Rate of evaporation decreases with the increase in humidity.
Explanation:
- The amount of water present in air is called humidity.
- The air around us cannot hold more than a definite amount of water vapour at a given temperature.
- If the amount of water vapour is high in air, then the rate of evaporation decreases. Ex : Clothes dry slowly on a rainy day than on a normal day.
![]()
(c) Explain the effect of wind speed on rate of evaporation.
Answer:
Rate of evaporation increases with the increase in wind speed.
Explanation:
- Because of increase in wind speed, particles of water vapour move away with the wind.
- Due to this, amount of water vapour in the surroundings decreases.
- It leads to increase in the rate of evaporation.
Ex: Clothes dry faster on a windy day or under fan than a normal day.